London Dispersion Force
London Dispersion Force: Easy to understand
Imagine you're at a crowded party, and everyone is evenly spread out, but suddenly, a famous celebrity walks in, drawing people closer to their side of the room and causing an uneven distribution. This is similar to how electrons, which are usually evenly spread around an atom, can suddenly shift and cluster on one side, creating a temporary imbalance. Just like the crowd at the party creates a temporary pull towards the celebrity, the clustered electrons create a temporary dipole, resulting in a weak attraction between molecules, known as the London dispersion force.
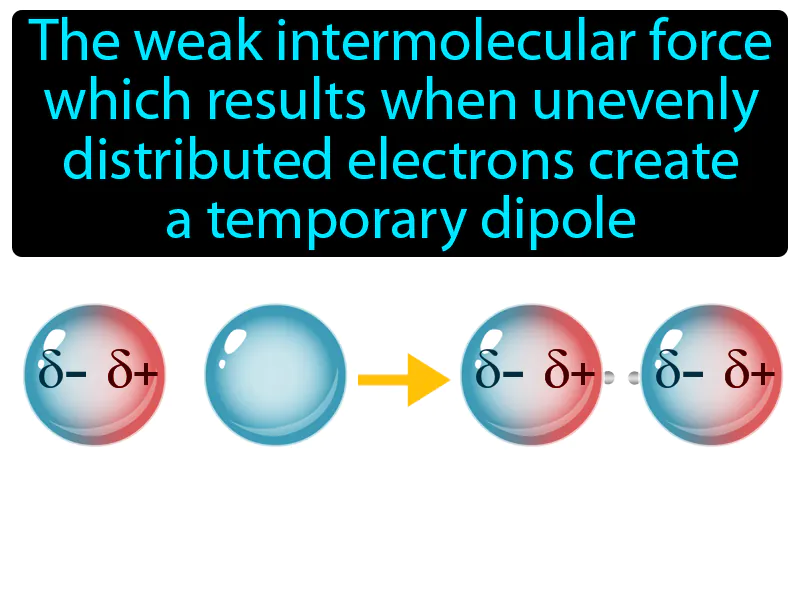
Practice Version
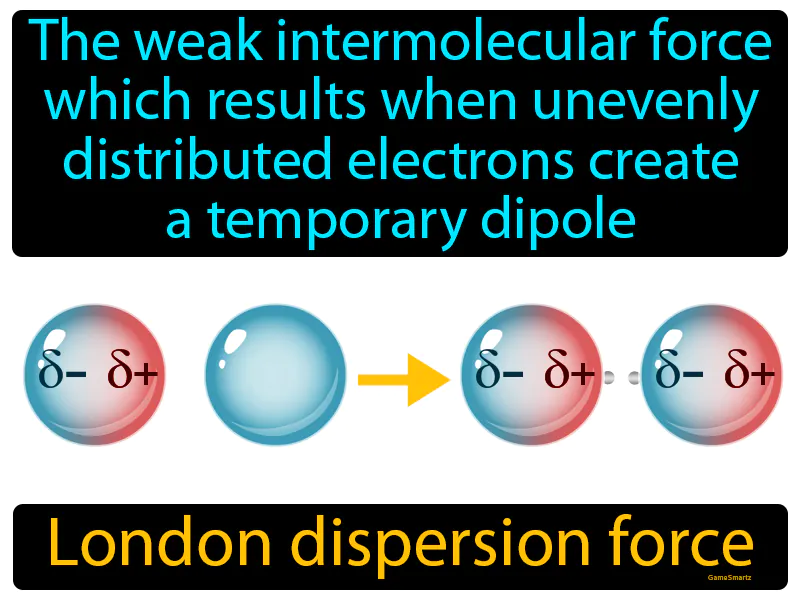
London Dispersion Force: The weak intermolecular force which results when unevenly distributed electrons create a temporary dipole. London dispersion force. London dispersion force is a temporary attractive force that occurs when electrons in adjacent atoms shift, causing a fleeting attraction.