Dipole-dipole Force
Dipole-dipole Force: Easy to understand
Imagine you're at a party, and you tend to gravitate towards people with similar interests, creating clusters of conversation. This is similar to how dipole-dipole forces work, where molecules with opposite charges on their ends are naturally drawn to each other. Just like you might be attracted to someone who shares your passion for movies while avoiding those who don't, molecules have regions with slight positive and negative charges that attract each other, leading to a natural alignment, much like forming groups at a social gathering based on shared interests.
Practice Version
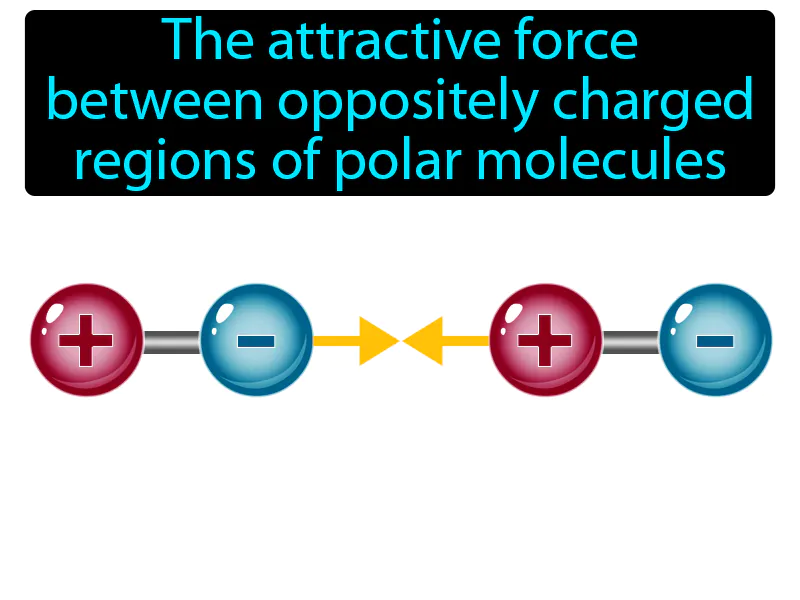
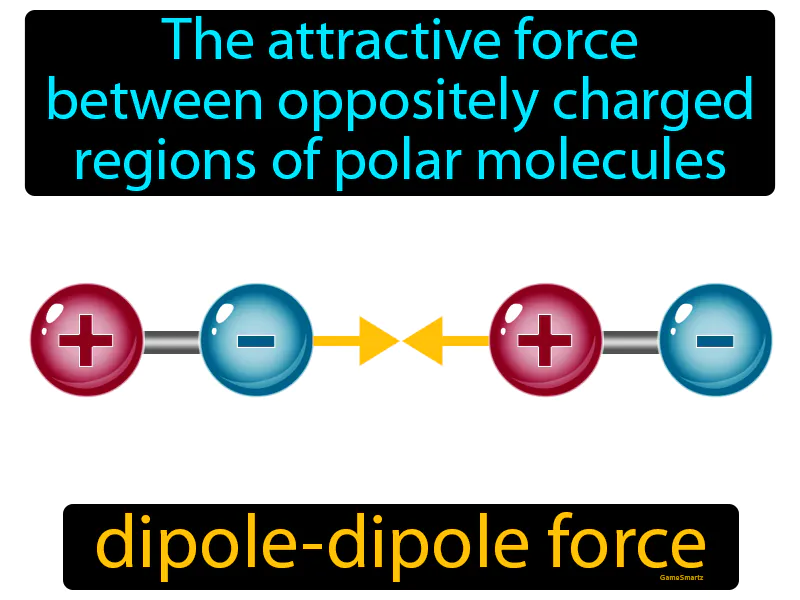
Dipole-dipole Force: The attractive force between oppositely charged regions of polar molecules. Dipole-dipole force. This force occurs when the positive end of one polar molecule attracts the negative end of another polar molecule.