Evaporation
What is Evaporation? Explained in an easy to understand way:

What's Covered in the Video:
Imagine sitting in a crowded room, and gradually people start leaving one by one, making the room feel less congested. This is similar to how molecules at the surface of a liquid gain enough energy to escape into the air as gas, reducing the overall density of the liquid at the top. Just like people leaving a crowded room makes it less crowded, molecules escaping from the surface of a liquid into the air make the liquid level decrease, illustrating the process of evaporation.
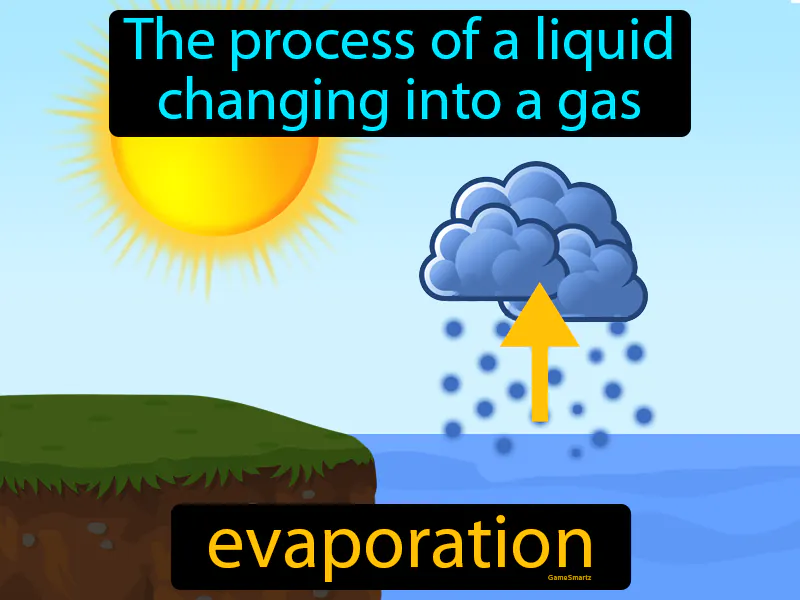
Practice Version
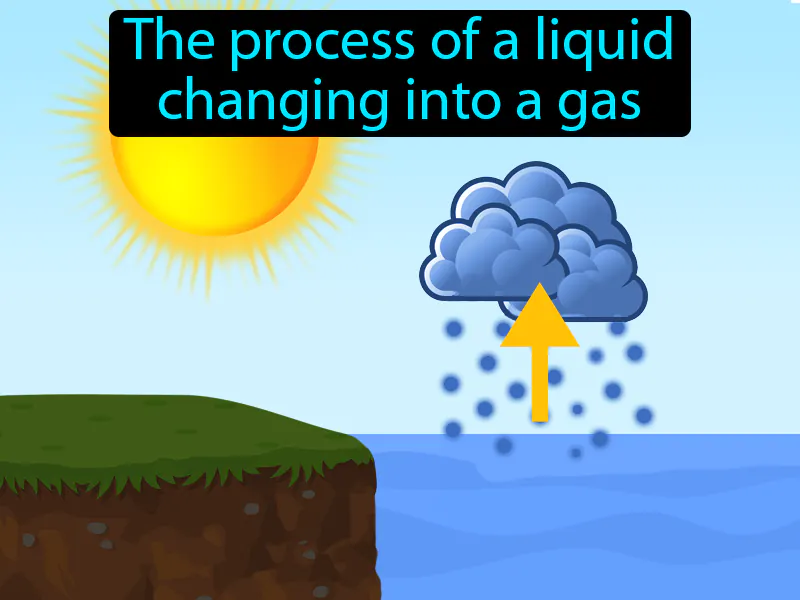
Evaporation: The top of liquid changing into a gas. Evaporation. Evaporation is when liquid water turns into water vapor and rises into the air.