Heterogeneous Reaction
Heterogeneous Reaction: Easy to understand
Imagine trying to have a conversation with someone on the other side of a glass wall. Just like how you and the other person are in two different spaces but still trying to communicate, a heterogeneous reaction involves reactants in different phases trying to interact and react with one another. In this analogy, the glass wall is like the boundary between phases (solid, liquid, or gas), and the conversation represents the chemical reaction taking place across that boundary.
Practice Version
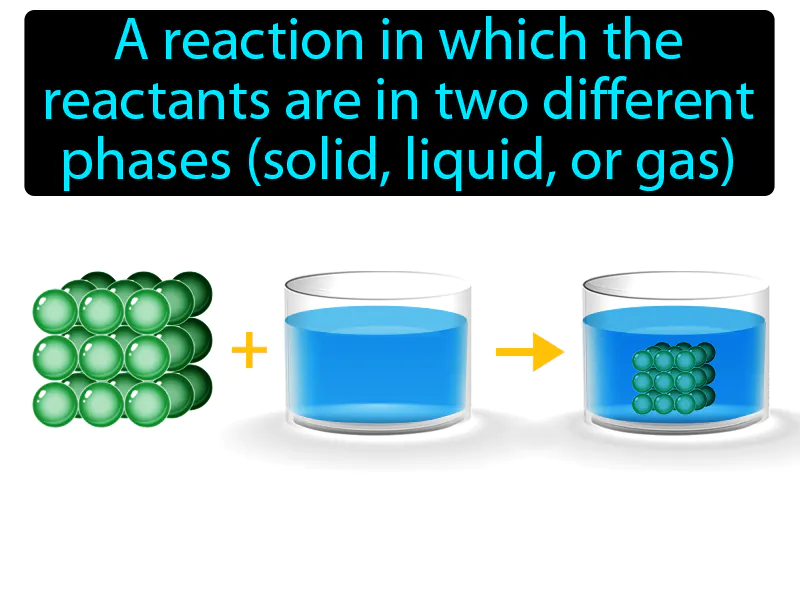
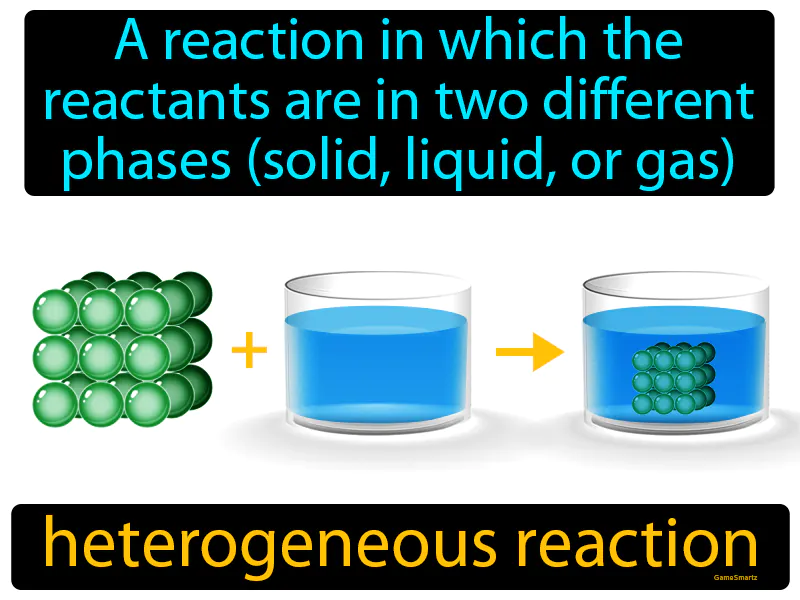
Heterogeneous Reaction: A reaction in which the reactants are in two different phases solid, liquid, or gas. Heterogeneous reaction. A heterogeneous reaction is when substances in different forms, like a solid and a liquid, react with each other.