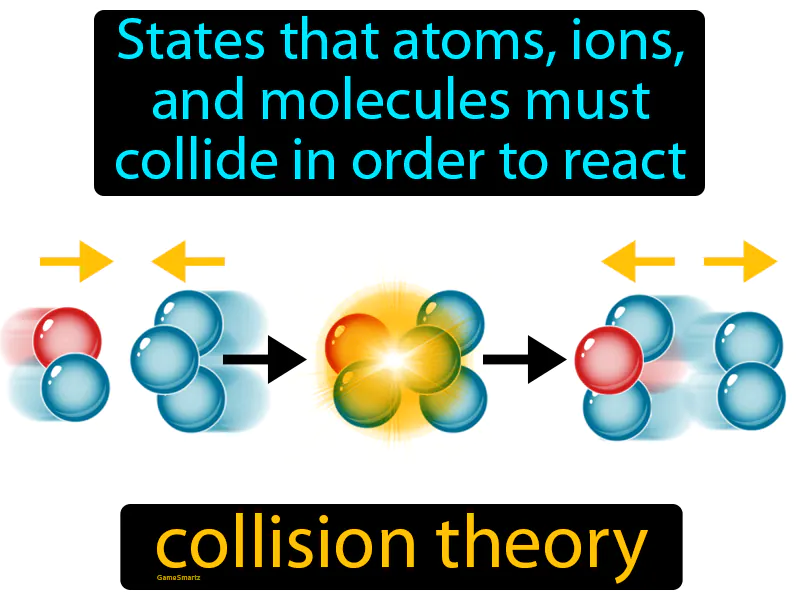
Collision Theory
Collision Theory: Easy to understand
Imagine trying to make new friends at a party; you can't just stand in a corner and expect connections to happen. Similarly, in collision theory, atoms, ions, and molecules must collide with each other to initiate a reaction. Just as interactions and conversations at the party are necessary to form friendships, collisions are necessary for chemical reactions to occur, where the energy and orientation during these collisions determine the success of the reaction, much like the quality of conversation affects friendship formation.
Practice Version
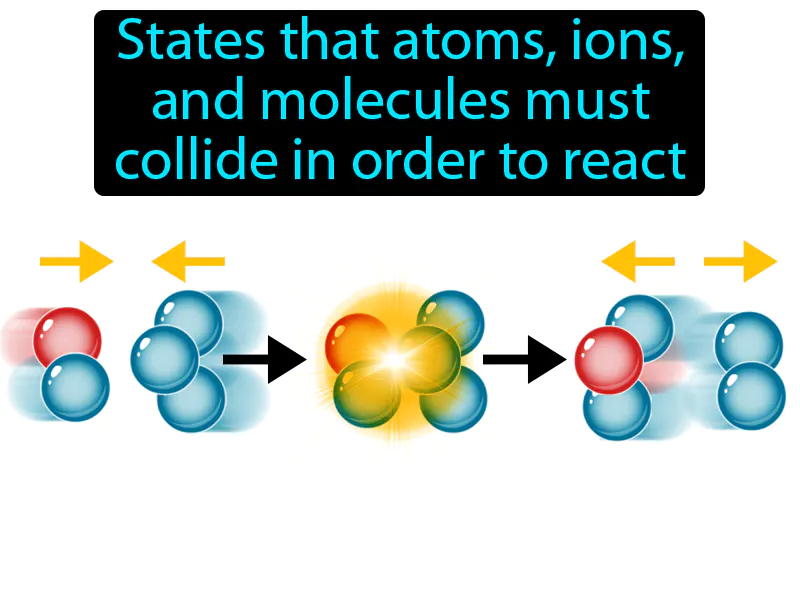
Collision Theory: States that atoms, ions, and molecules must collide in order to react. Collision theory. In simple terms, collision theory explains that chemical reactions occur when particles crash into each other with enough energy and the right orientation.