Rate Law
Rate Law: Easy to understand
Imagine you're trying to fill a bathtub quickly to take a bath. The speed at which the bathtub fills up depends on how wide open the faucet is, much like how the rate of a chemical reaction depends on the concentration of its reactants. Just as the water flow rate increases when you turn the faucet more, increasing the concentration of reactants can speed up the reaction rate. In both cases, there’s a direct relationship between the input (faucet opening or reactant concentration) and the output (water filling speed or reaction rate), highlighting how the rate law links reaction speed to reactant levels.
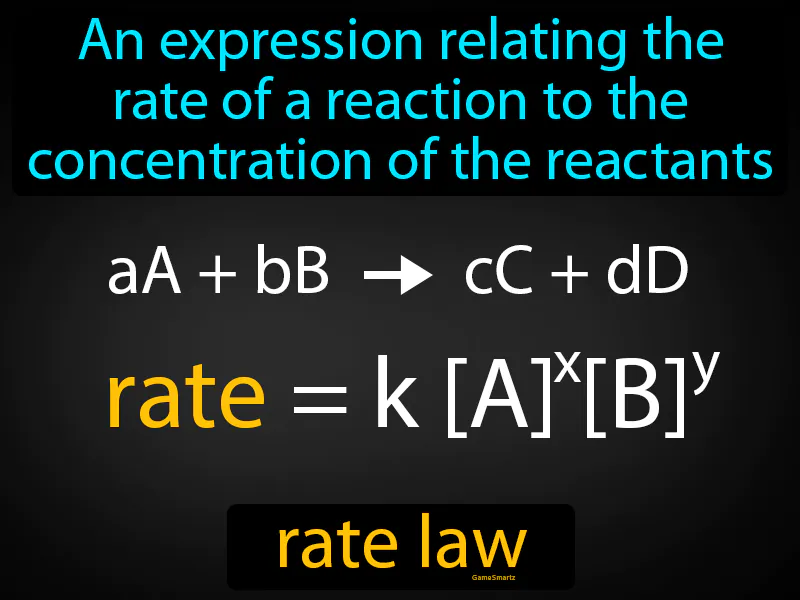
Practice Version
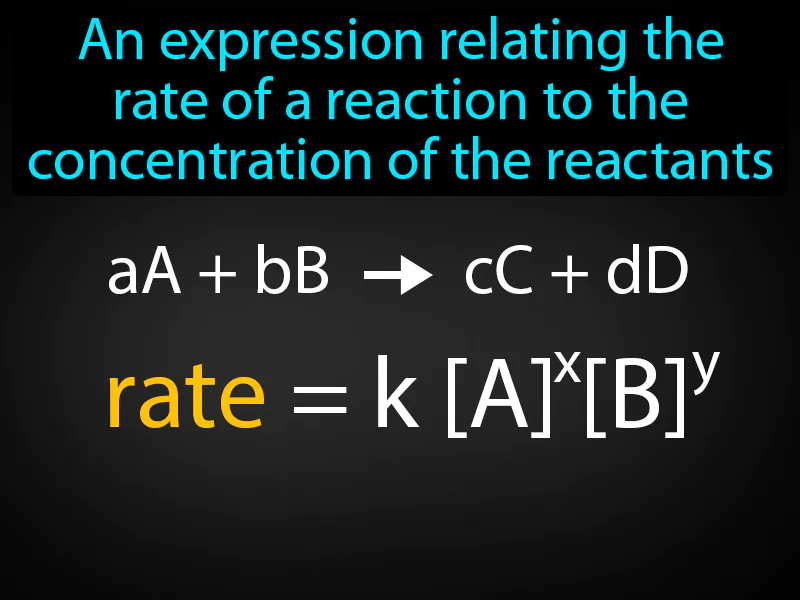
Rate Law: An expression relating the rate of a reaction to the concentration of the reactants. Rate law. Rate law is a mathematical equation that shows how the speed of a chemical reaction depends on the amount of reactants present.