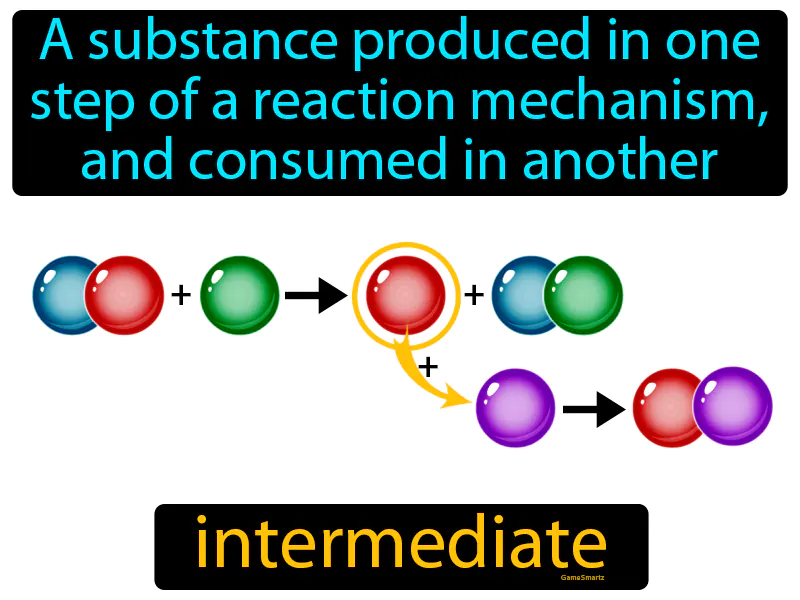
Intermediate
Intermediate: Easy to understand
Imagine you're planning a road trip and need to make a stop at a gas station to refuel before continuing your journey. This is similar to an intermediate in a chemical reaction, where a substance is created at one stage and used up in the next to ensure the process continues smoothly. In this analogy, the gas station acts like the intermediate: it's not your final destination, but a necessary step that supports your journey just like intermediates support the progression of chemical reactions by being formed and then consumed.
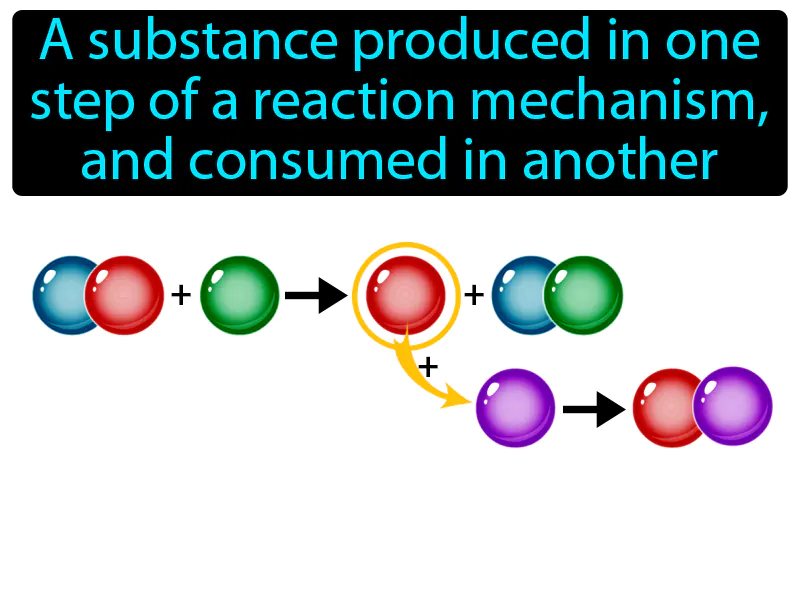
Practice Version
Intermediate: A substance produced in one step of a reaction mechanism, and consumed in another, is called an intermediate. In Science, an intermediate is a temporary substance formed during the steps of a chemical reaction.