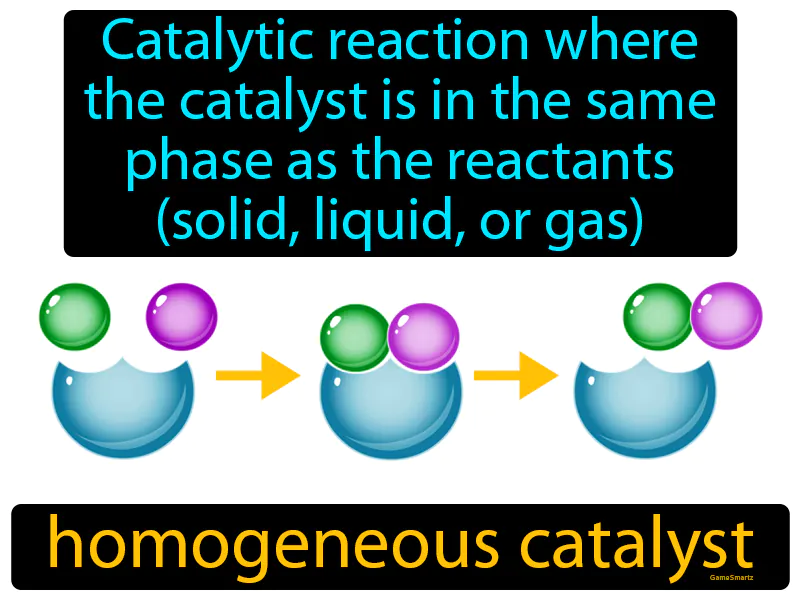
Homogeneous Catalyst
Homogeneous Catalyst: Easy to understand
Imagine trying to untangle a bunch of necklaces that are all mixed up in the same jewelry box. Just like using a pair of nimble fingers already inside the box to sort out the mess, a homogeneous catalyst operates in the same phase as the reactants to streamline the reaction process. In this analogy, the tangled necklaces represent the reactants, the fingers are the catalyst, and the jewelry box is the shared phase, all working together seamlessly to achieve the desired outcome.
Practice Version
Homogeneous Catalyst: Catalytic reaction where the catalyst is in the same phase as the reactants, solid, liquid, or gas. Homogeneous catalyst. A homogeneous catalyst is a substance that speeds up a chemical reaction while being in the same physical state as the reactants.