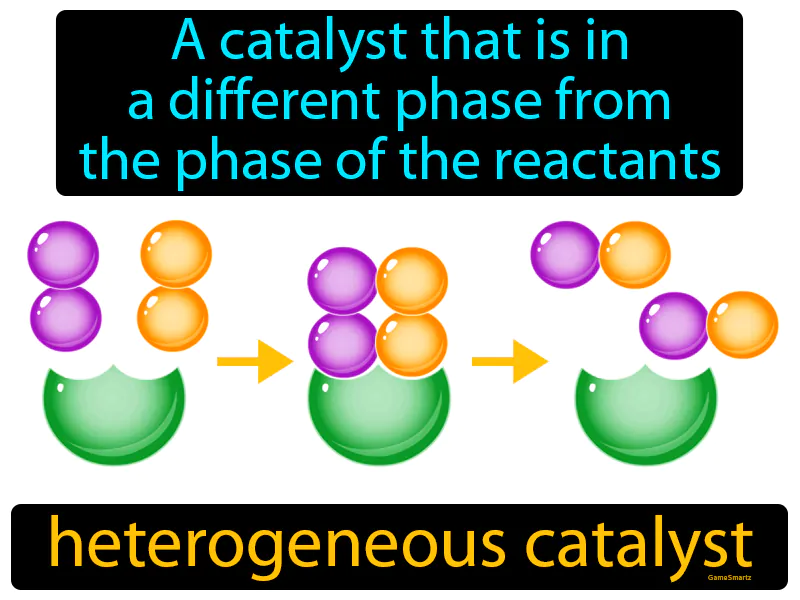
Heterogeneous Catalyst
Heterogeneous Catalyst: Easy to understand
Imagine you're trying to find your keys lost in a cluttered room. Just like a magnet can help you find a metal key among a pile of non-magnetic clutter, a heterogeneous catalyst helps speed up a chemical reaction by being in a different phase than the reactants, thereby making it easier to interact with them. The magnet represents the solid-phase catalyst, while the metal keys represent the reactants in a different phase, such as a gas or liquid, with the magnet's ability to attract and assist in organizing or finding the keys mirroring how the catalyst interacts with reactants to facilitate the reaction.
Practice Version
Heterogeneous Catalyst: A catalyst that is in a different phase from the phase of the reactants. Heterogeneous catalyst. A heterogeneous catalyst speeds up a chemical reaction while being in a different state, like a solid catalyst working with gas reactants.