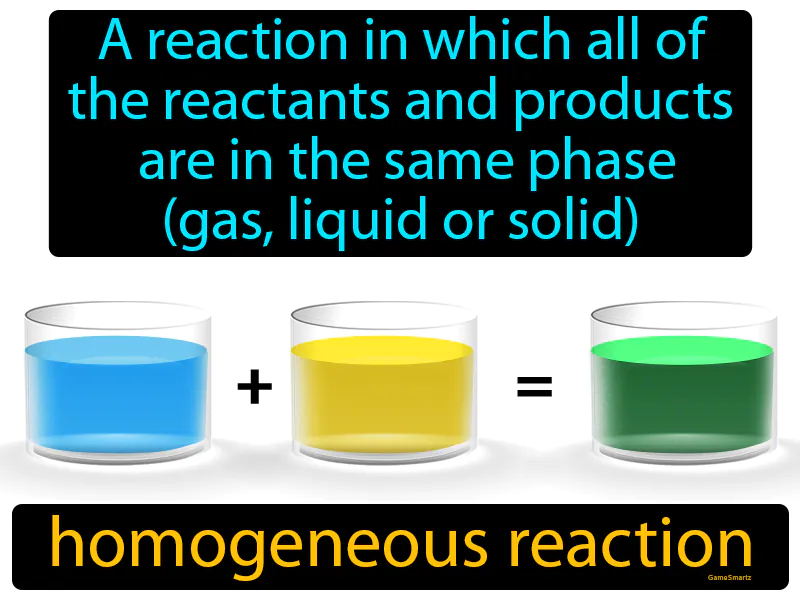
Homogeneous Reaction
Homogeneous Reaction: Easy to understand
Imagine trying to make a smoothie where all the ingredients must be blended into one uniform mixture. Just like how you combine fruits and yogurt all in the liquid phase to create a smoothie, a homogeneous reaction involves reactants and products all existing in the same phase, whether gas, liquid, or solid. In both cases, everything starts in the same phase to ensure a smooth, consistent result, much like how the fruits and yogurt must all be liquids to blend seamlessly into a delicious drink.
Practice Version

Homogeneous Reaction: A reaction in which all of the reactants and products are in the same phase gas, liquid, or solid is called a homogeneous reaction. A homogeneous reaction occurs when substances mix uniformly in a single phase, like gases or liquids reacting together.