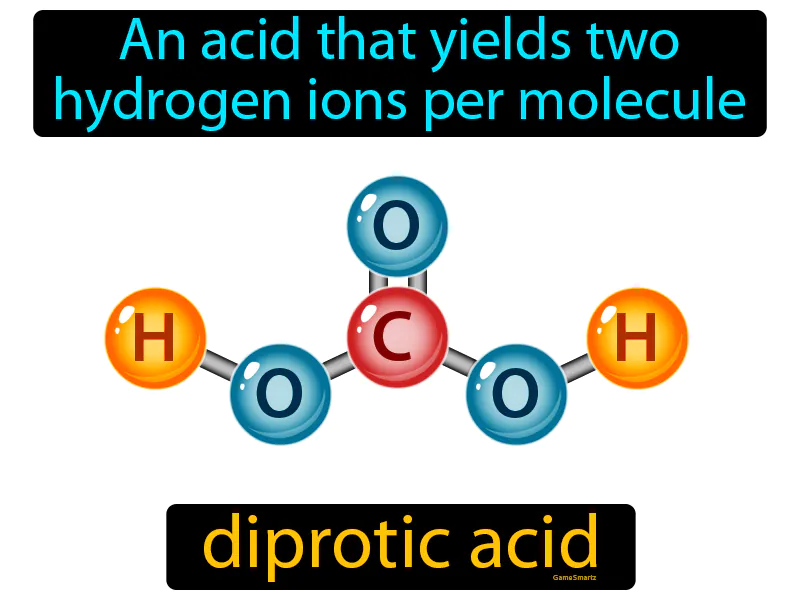
Diprotic Acid
Diprotic Acid: Easy to understand
Imagine you're carrying a carton of eggs home from the grocery store, and it accidentally drops, spilling more than one egg onto the ground. This scenario is similar to a diprotic acid, which releases two hydrogen ions per molecule when it dissociates in solution. Just as the carton can release multiple eggs when dropped, a diprotic acid can release two hydrogen ions, each representing an egg, as it breaks apart in water.
Practice Version
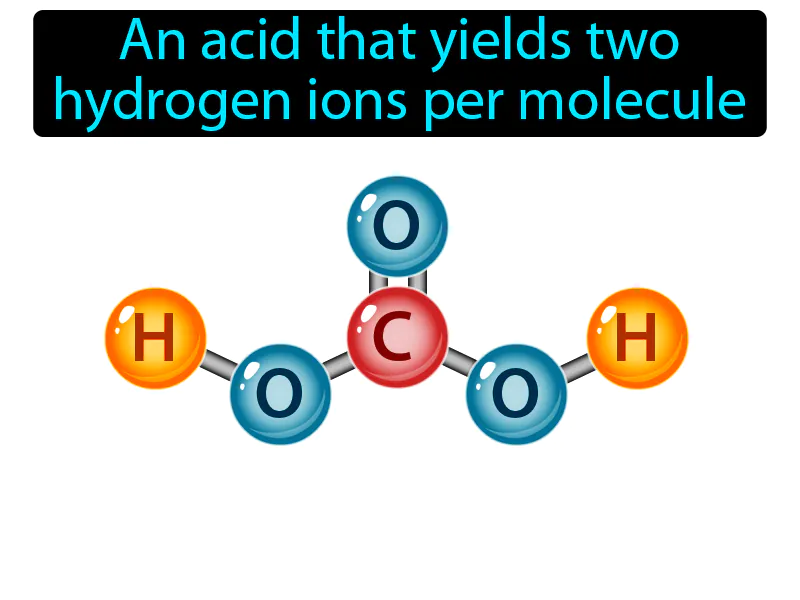
Diprotic Acid: An acid that yields two hydrogen ions per molecule. Diprotic acid. A diprotic acid can give away two hydrogen ions when it reacts with other substances.