Acid Ionization Constant
Acid Ionization Constant: Easy to understand
Imagine trying to decide how to distribute limited seating at a popular restaurant among various groups of people. This situation is similar to how an acid decides how much of its protons to give away to water molecules in a solution. Just as the restaurant must balance seating arrangements to maintain an optimal flow and keep everyone satisfied, the acid must reach an equilibrium where the rate of giving away protons equals the rate of reclaiming them, which is quantified by the acid ionization constant.

Practice Version
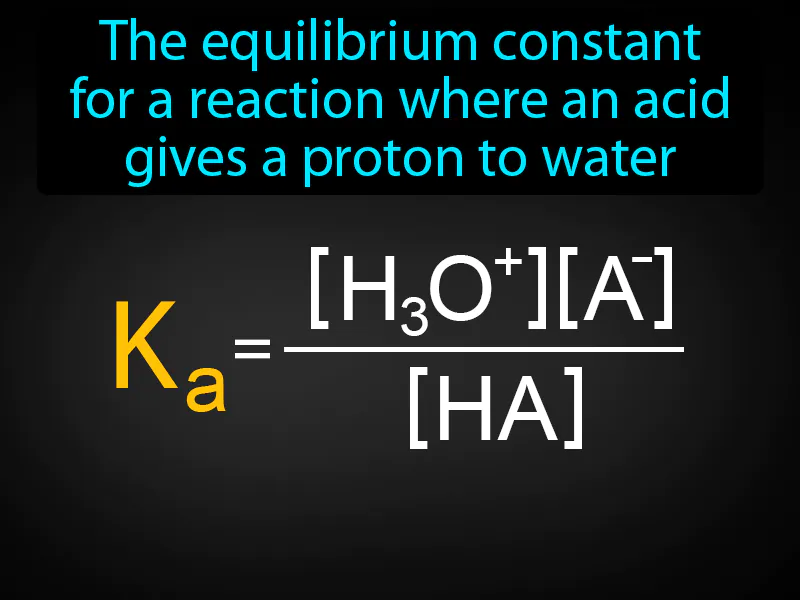
Acid Ionization Constant: The equilibrium constant for a reaction where an acid gives a proton to water. Acid ionization constant. It measures how well an acid releases protons in water, indicating its strength.