Strong Acid
Strong Acid: Easy to understand
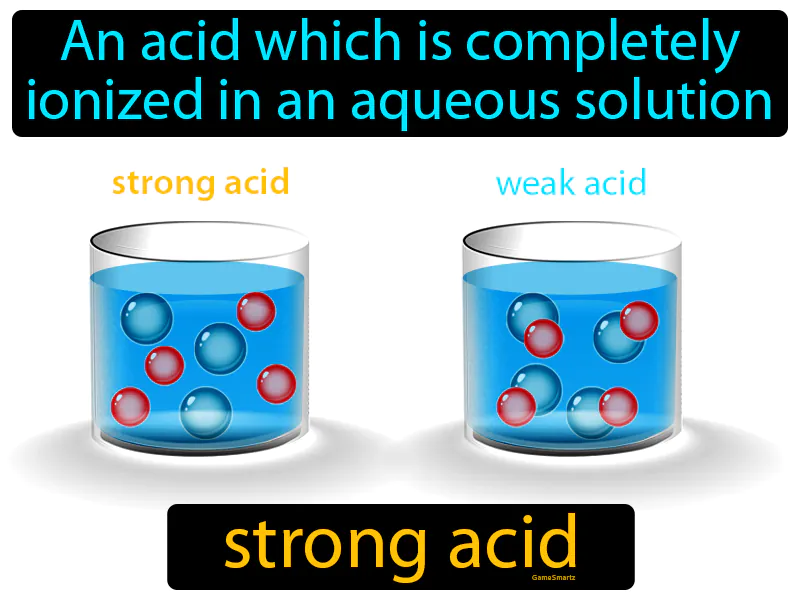
Imagine you're at a crowded party, and suddenly the host opens up all the doors and windows, letting everyone scatter freely into the garden. This scenario is similar to how a strong acid behaves in water, where the acid molecules completely dissociate into ions, just like party-goers rushing out into the open space. In this analogy, the crowded party represents the concentrated state of the acid, the opening of doors and windows symbolizes the introduction of water, and the people dispersing are akin to the acid molecules fully ionizing, spreading uniformly throughout the solution.
Practice Version
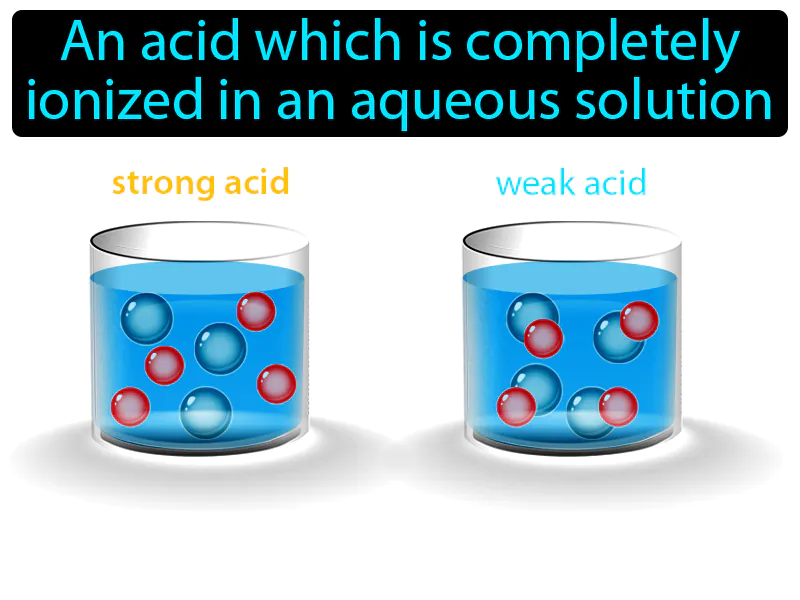
Strong Acid: An acid which is completely ionized in an aqueous solution. Strong acid. A strong acid is one that breaks apart fully in water, releasing lots of hydrogen ions.