Arrhenius Model
Arrhenius Model: Easy to understand
Imagine you've just spilled salt into your coffee instead of sugar, drastically changing the taste of your morning brew. Just as the salt transforms the flavor of your coffee by introducing new elements, the Arrhenius model explains how acids and bases alter the properties of water by introducing hydrogen ions and hydroxide ions, respectively. In this analogy, the salt represents an acid, adding hydrogen ions (new flavors) to the coffee (water), while sugar could be seen as a base, introducing hydroxide ions, each fundamentally changing the solution's character.
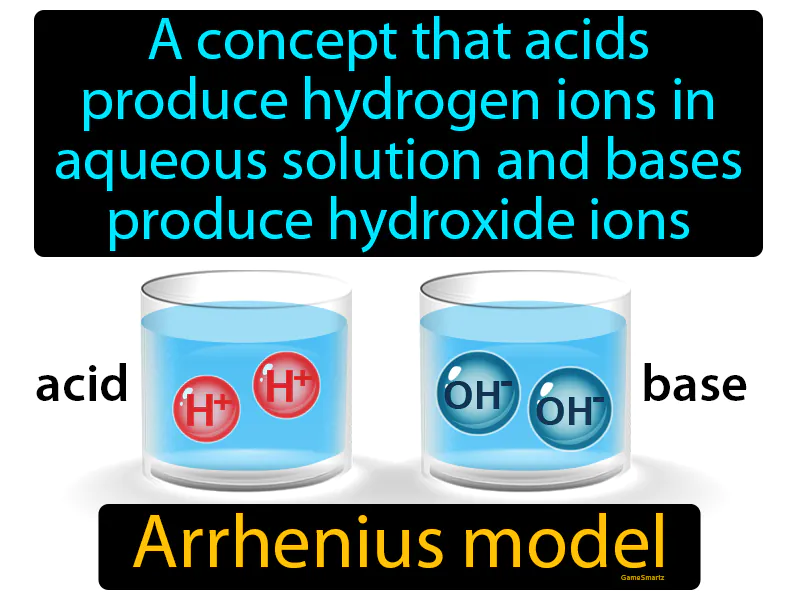
Practice Version
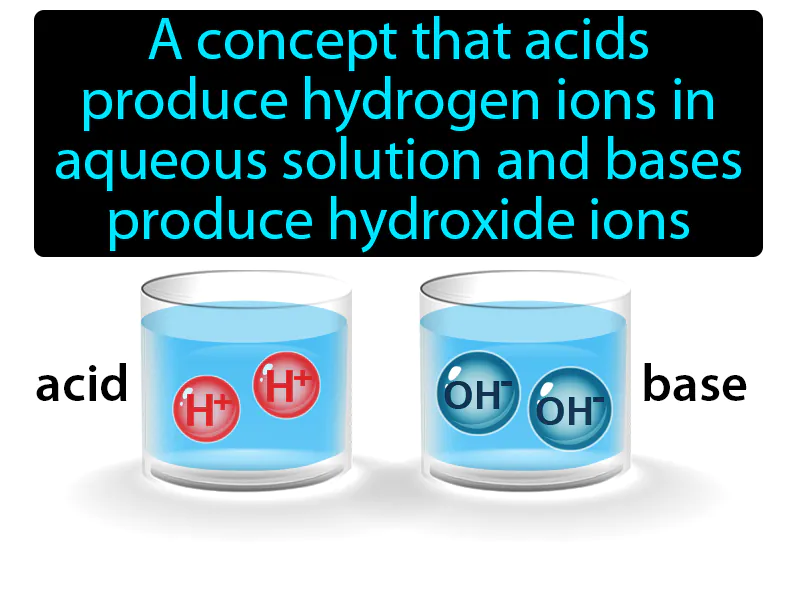
Arrhenius Model: A concept that acids produce hydrogen ions in aqueous solution and bases produce hydroxide ions. Arrhenius model. The Arrhenius model explains how acids and bases behave in water by releasing specific ions.