Ion-product Constant
Ion-product Constant: Easy to understand
Imagine trying to maintain a stable room temperature when people keep opening and closing doors, causing fluctuations. This is similar to how water constantly self-ionizes, balancing the concentration of hydrogen ions and hydroxide ions, much like how the room attempts to maintain a consistent temperature despite external changes. Just as the room's temperature has a specific point it returns to for stability, the ion-product constant of water (Kw) is the equilibrium point where the concentrations of ions stabilize, ensuring a consistent balance despite ongoing shifts.
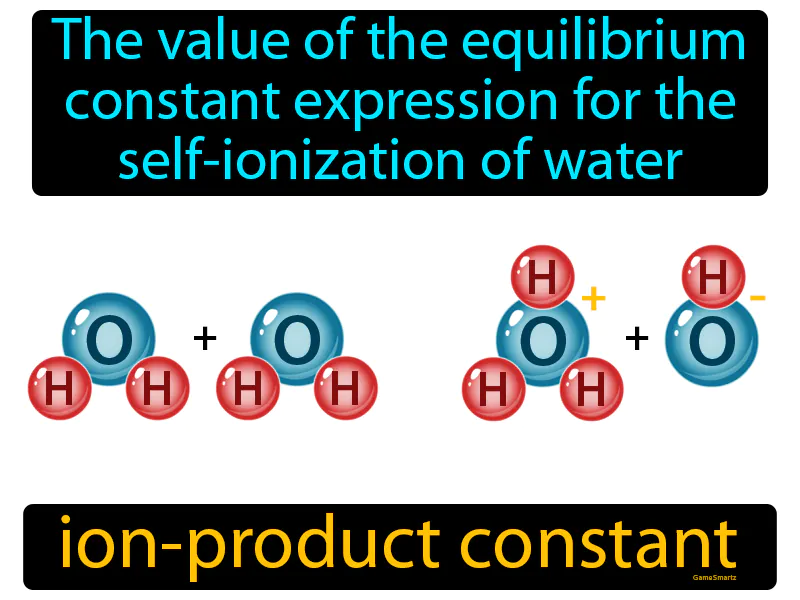
Practice Version
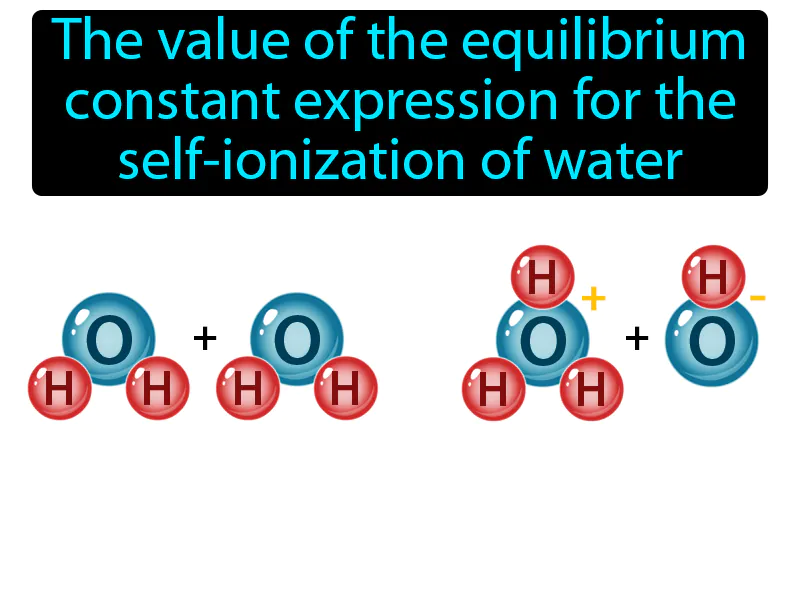
Ion-product Constant: The value of the equilibrium constant expression for the self-ionization of water. Ion-product constant. The ion-product constant is the product of the concentrations of hydrogen ions and hydroxide ions in water, which remains constant at a given temperature.