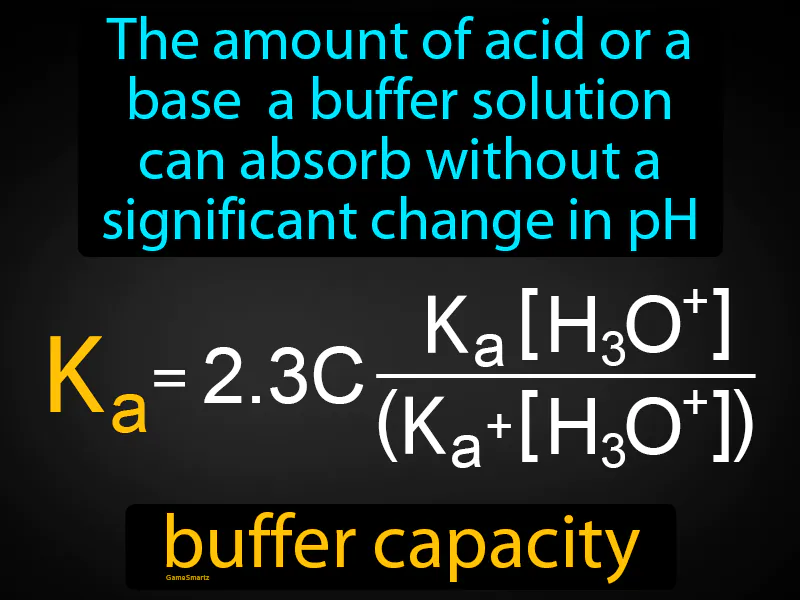
Buffer Capacity
Buffer Capacity: Easy to understand
Imagine you're at a large party where the room can comfortably handle a certain number of people before it becomes overcrowded. This is like a buffer solution, which can absorb a certain amount of acid or base before the pH changes significantly. Just as the room can accommodate more guests without becoming uncomfortable up to a point, a buffer can neutralize added acids or bases without a major shift in pH, maintaining a stable environment until its capacity is exceeded.
Practice Version

Buffer Capacity: The amount of acid or a base a buffer solution can absorb without a significant change in pH. Buffer capacity. Buffer capacity is the ability of a solution to resist changes in pH when acids or bases are added.