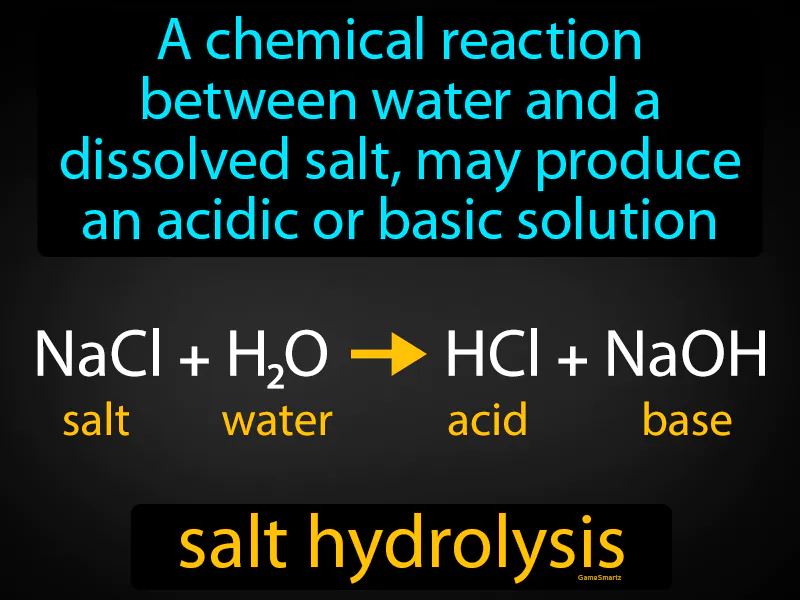
Salt Hydrolysis
Salt Hydrolysis: Easy to understand
Imagine you're trying to balance the flavors in a soup, but adding certain spices can either make it too spicy or too bland. This is similar to salt hydrolysis, where a dissolved salt interacts with water and can create a solution that is either acidic or basic. Just as the right mix of spices can shift the overall taste of the soup, the components of the salt and their reaction with water can shift the pH of the solution, determining whether it becomes more acidic or basic.
Practice Version
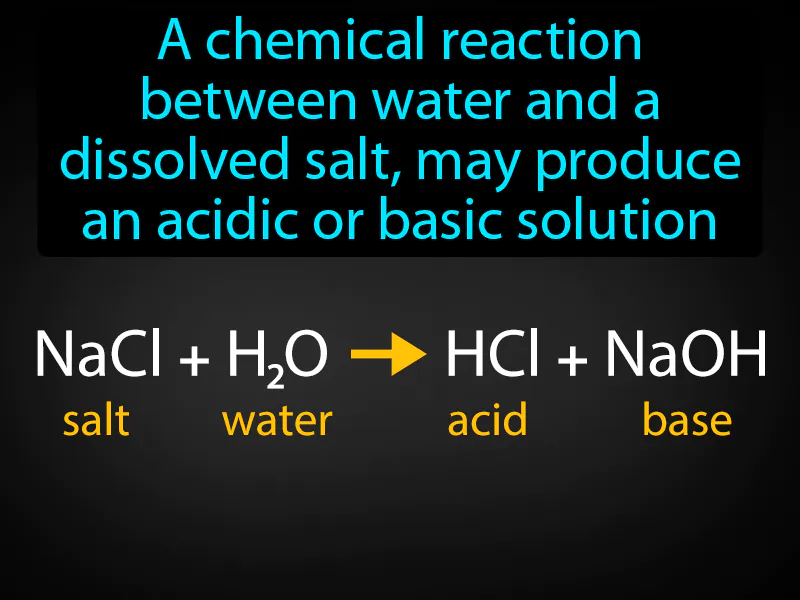
Salt Hydrolysis: A chemical reaction between water and a dissolved salt may produce an acidic or basic solution. Salt hydrolysis is when the ions from a dissolved salt react with water to form an acidic or basic solution.