Weak Base
Weak Base: Easy to understand
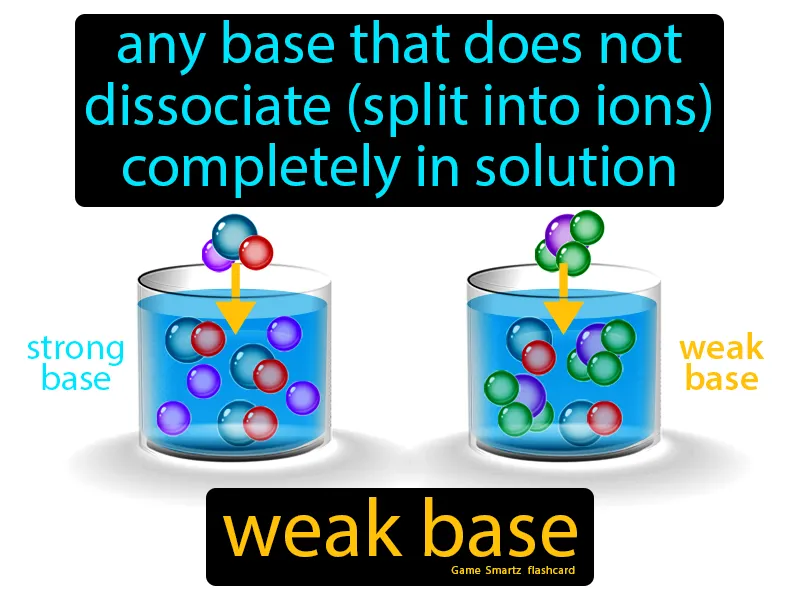
Imagine trying to get a group of friends to agree on a place to eat, but only a few actually voice their preferences while the rest remain undecided. This situation is similar to a weak base in chemistry, where only a small fraction of the molecules dissociate into ions in solution. Just as in the dining dilemma, where not everyone participates in the decision, a weak base doesn't fully dissociate, meaning only a limited number of its molecules break apart and engage in reactions in the solution.
Practice Version
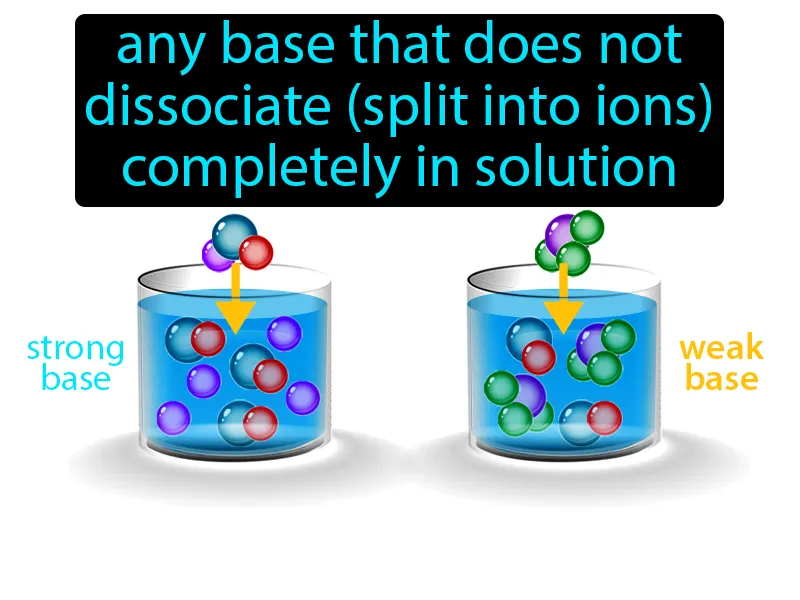
Weak Base: Any base that does not dissociate split into ions completely in solution. Weak base. A weak base is a substance that only partially ionizes in water, resulting in fewer available ions.