Ideal Gas Law
Ideal Gas Law: Easy to understand
Imagine trying to pack your suitcase for a trip, and it seems like no matter how you arrange things, you can't close it comfortably. This is similar to the ideal gas law, where the pressure (P) inside your suitcase increases as you try to fit more items (increasing volume, V) at a constant temperature (T), making it harder to close. Just like how adding more clothes (items) requires more space or force to close the suitcase (pressure), in the ideal gas law, increasing the amount of gas (n) or temperature (T) affects the pressure and volume relationship, much like trying to fit everything without exceeding the suitcase's capacity.

Practice Version
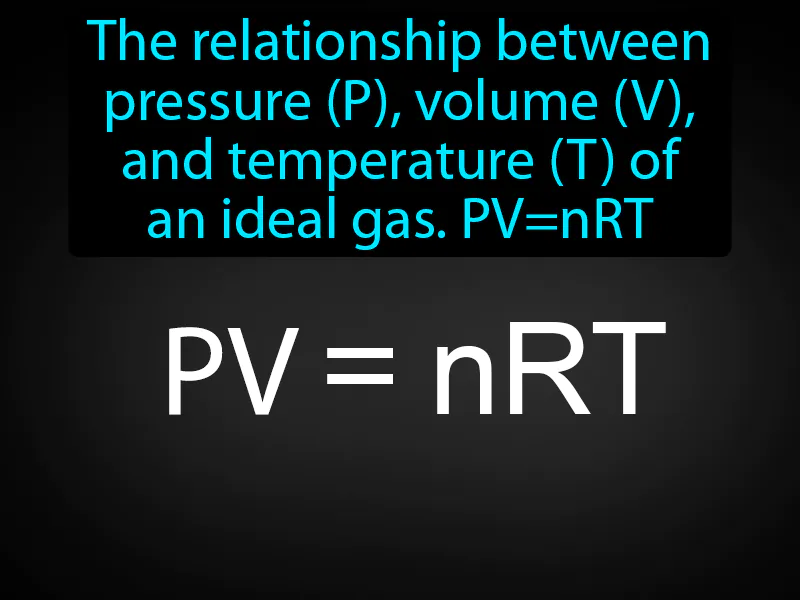
Ideal Gas Law: The relationship between pressure P, volume V, and temperature T of an ideal gas PVnRT. Ideal gas law. The ideal gas law is a formula that describes how the pressure, volume, and temperature of a gas are related, assuming the gas behaves perfectly.