Boyles Law
What is Boyle's Law? Explained in an easy to understand way:
What's Covered in the Video:
Imagine you're packing a suitcase for a long trip and trying to fit all your clothes into a limited space. As you push down to compress the clothes (decreasing their volume), you feel the suitcase getting harder to close because of the increased pressure inside. Similarly, Boyle's Law describes how, when the volume of a gas decreases, its pressure increases, given that the temperature remains constant. Just like squeezing more clothes into a suitcase makes it harder to close due to increased pressure, compressing a gas into a smaller volume results in increased pressure if the temperature doesn't change.
Practice Version
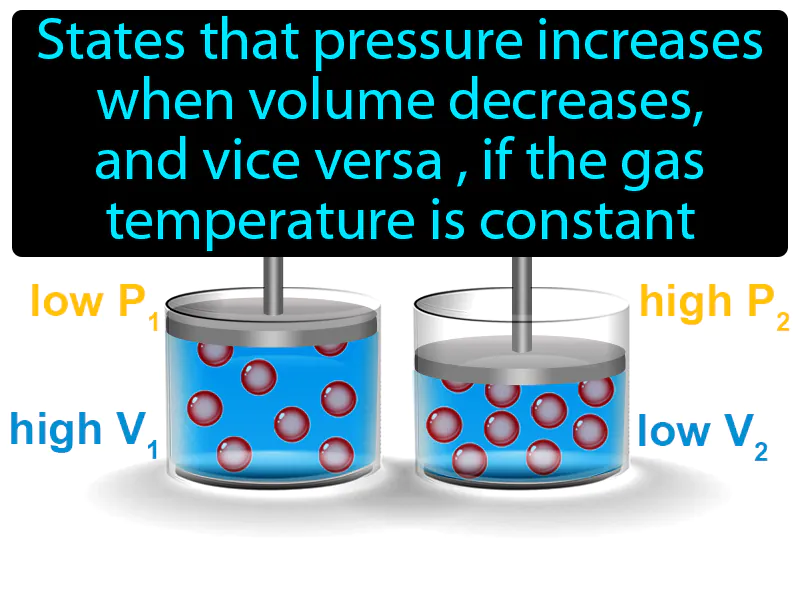
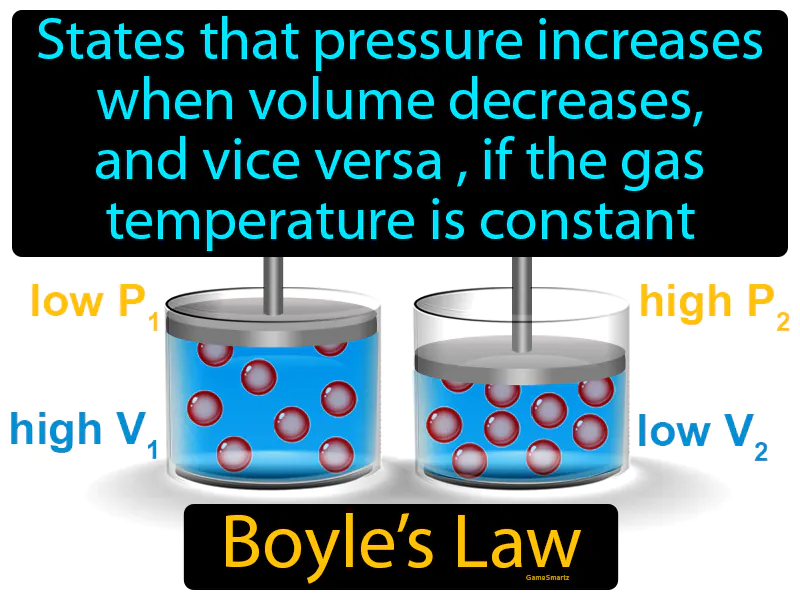
Boyles Law: States that pressure increases when volume decreases, and vice versa, if the gas temperature is constant. Boyle's Law It describes how gas pressure and volume are inversely related at constant temperature.