Grahams Law Of Effusion
Grahams Law Of Effusion: Easy to understand
Imagine you're trying to catch a bus across town, but you have a heavy backpack weighing you down. Just like it's harder for you to move quickly with a heavy bag, a gas with a higher molar mass moves more slowly through a small opening. The connection here is that your speed is limited by the weight you carry, much like how the rate of effusion of a gas is slower when its molar mass is larger, demonstrating the inverse relationship in Graham's law.
Practice Version
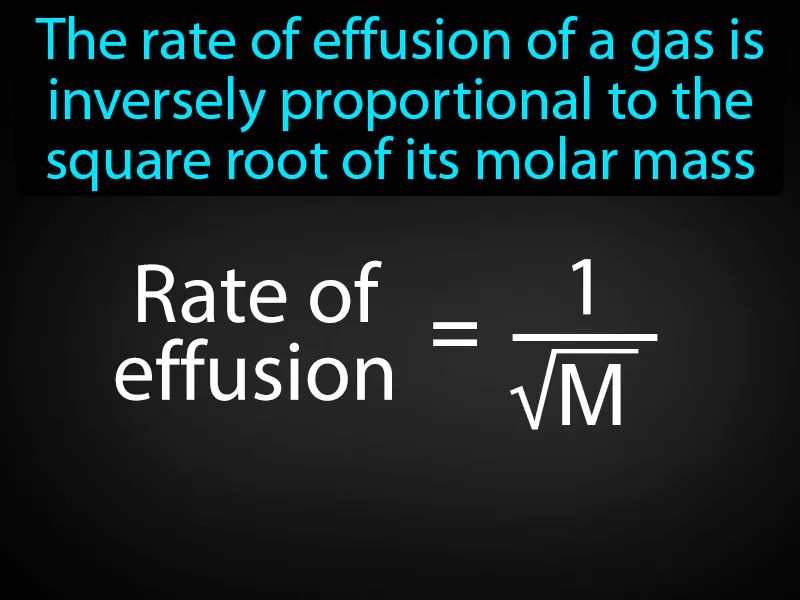
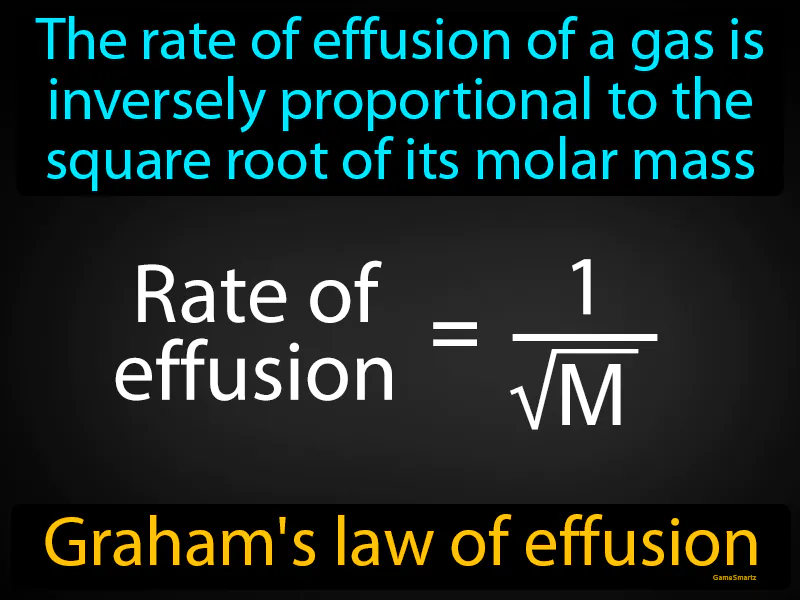
Grahams Law Of Effusion: The rate of effusion of a gas is inversely proportional to the square root of its molar mass. Graham's law of effusion states that lighter gases escape through a tiny opening faster than heavier gases.