Daltons Law Of Partial Pressures
Daltons Law Of Partial Pressures: Easy to understand
Imagine trying to carry a bunch of grocery bags from your car to your kitchen in one trip. Each bag represents a different item, like cereal, milk, or vegetables, and together they create the total weight you feel in your arms. Just like each bag adds its weight to the total load you're carrying, in Dalton's law, each gas in a mixture contributes its pressure to the total pressure, much like each bag contributes to the total weight of groceries.
Practice Version

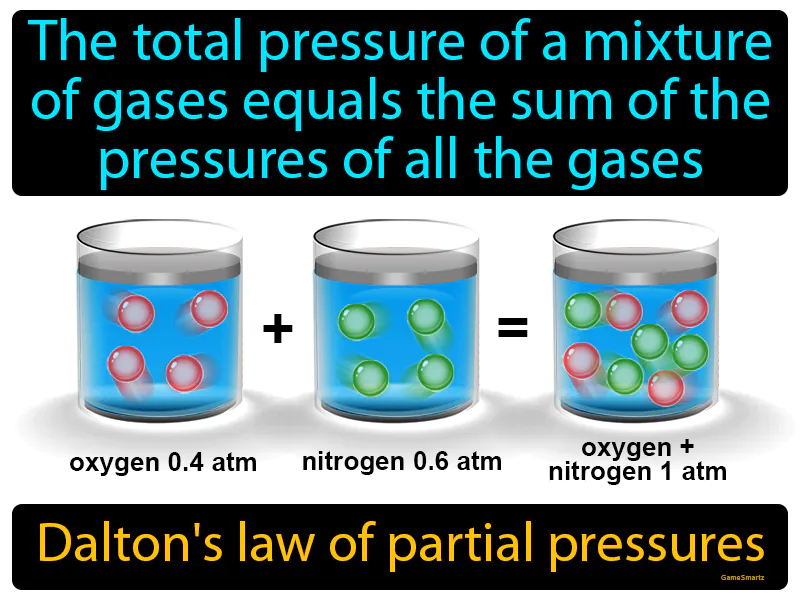
Daltons Law Of Partial Pressures: The total pressure of a mixture of gases equals the sum of the pressures of all the gases. Dalton's law of partial pressures. Dalton's law states that in a mixture of gases, each gas contributes to the total pressure independently of the others.