Molar Volume
Molar Volume: Easy to understand
Imagine trying to pack a suitcase for a trip, and you're struggling to fit all your clothes in a limited space. Just like how you need to know how much space is available in your suitcase to pack efficiently, scientists use the concept of molar volume to understand how much space one mole of gas occupies under specific conditions. In this analogy, the suitcase is like the volume of 22.4 liters, the clothes represent the one mole of gas, and the airline's baggage policies are akin to the conditions of 1 atm pressure and 0°C temperature, which dictate the space that the gas will fill.
Practice Version

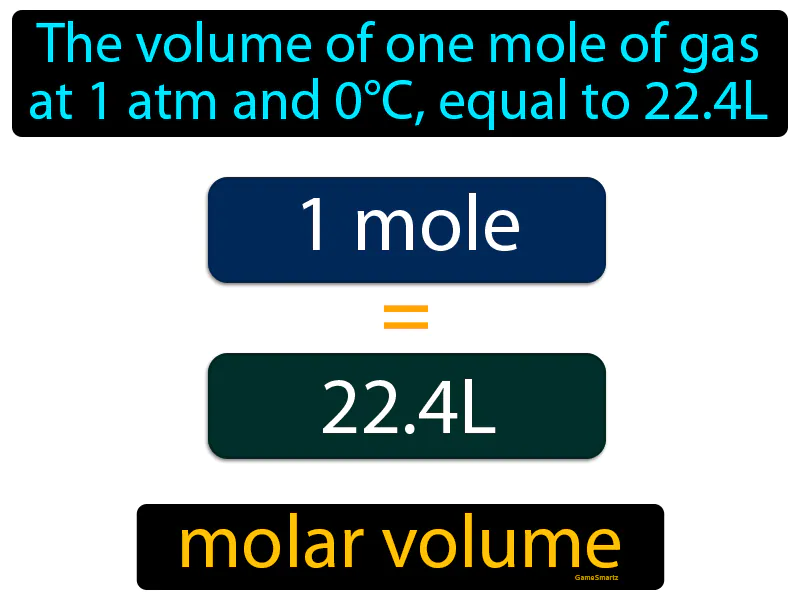
Molar Volume: The volume of one mole of gas at 1 atm and 0C, equal to 22.4L, is known as molar volume. Molar volume is the space one mole of gas occupies under specific conditions.