Combined Gas Law
Combined Gas Law: Easy to understand
Imagine trying to fit all your clothes into a suitcase for a trip, only to realize they won't all fit unless you adjust how you pack them. This situation is akin to the combined gas law, where pressure, temperature, and volume must be adjusted to fit specific conditions. Just as rearranging clothes affects how much space they take up (volume), how tightly they're packed (pressure), and the flexibility of the suitcase fabric (temperature), the combined gas law shows how adjusting one factor requires changes in the others to maintain balance in a closed system.
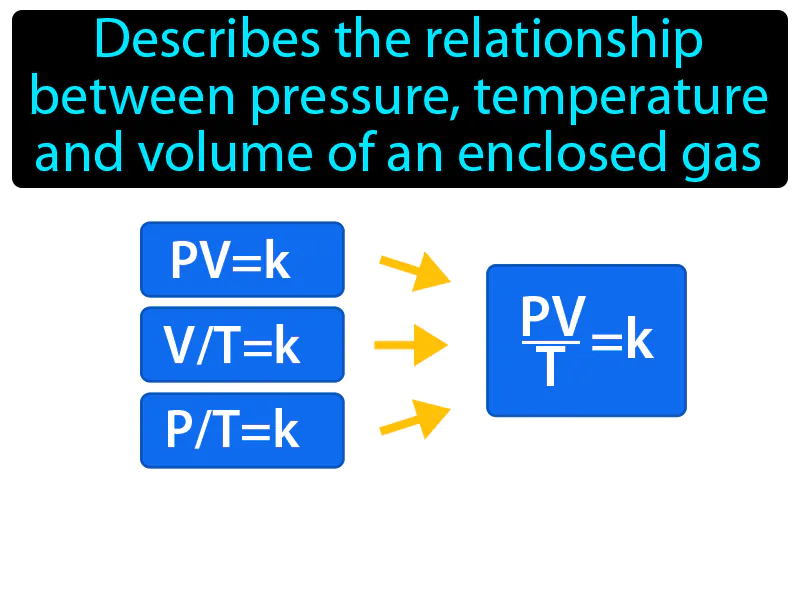
Practice Version
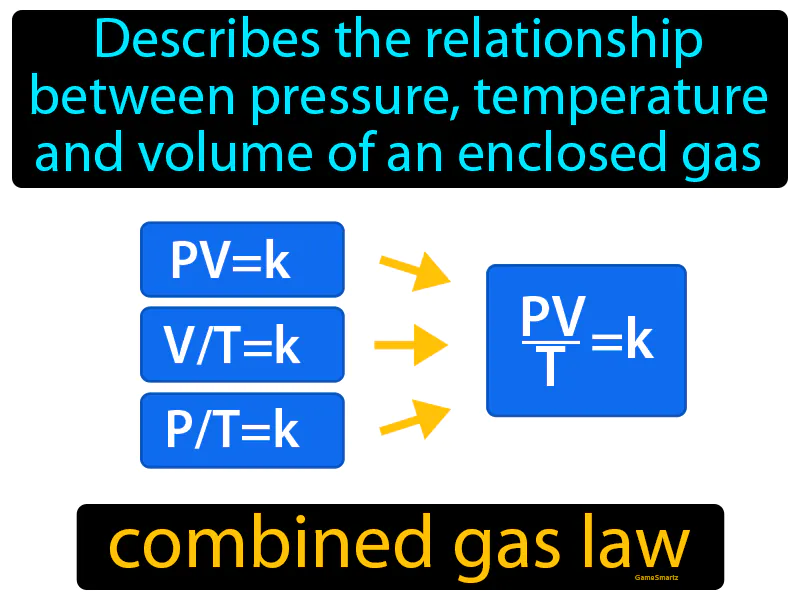
Combined Gas Law: Describes the relationship between pressure, temperature and volume of an enclosed gas. Combined gas law. It shows how these three properties of a gas change together when conditions change, keeping the amount of gas constant.