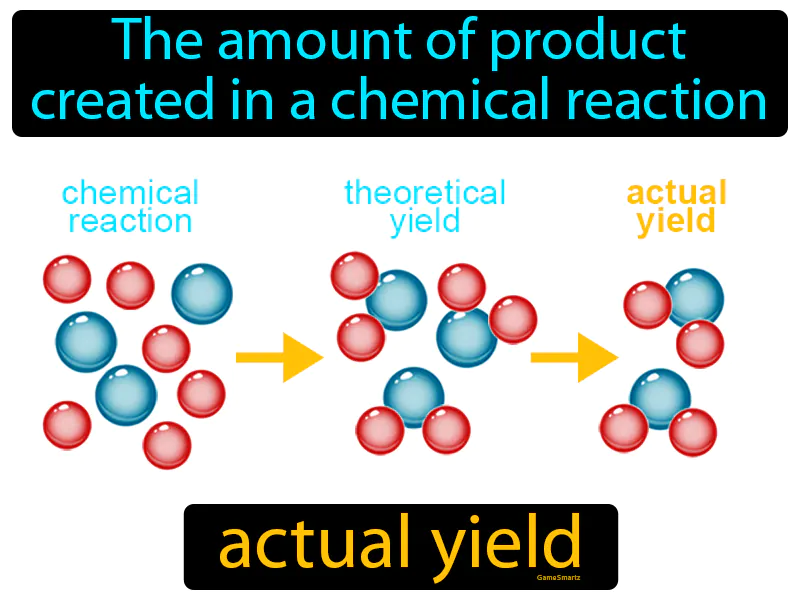
Actual Yield
Actual Yield: Easy to understand
Imagine you're baking a batch of cookies, expecting to make 24 cookies, but you only end up with 18 because some dough was lost to taste-testing or sticking to the bowl. This situation is similar to the concept of actual yield in chemistry, where the amount of product you obtain from a reaction is often less than what you theoretically expected. Just like the cookies, where the anticipated 24 represents the theoretical yield, the 18 cookies you actually baked represent the actual yield in a chemical reaction, highlighting that real-world processes often involve some loss or inefficiency.
Practice Version
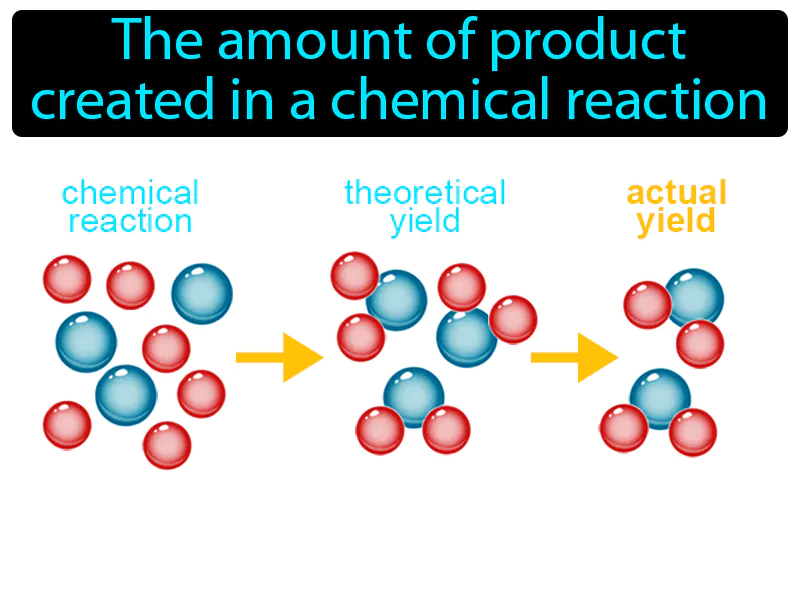
Actual Yield: The amount of product created in a chemical reaction. Actual yield. Actual yield is the measured amount of a product obtained from a chemical reaction.