Mole Ratio
Mole Ratio: Easy to understand
Imagine you're preparing a batch of cookies and the recipe specifies you need 2 cups of flour for every 1 cup of sugar. This is similar to a chemical equation where substances react in specific ratios, much like ingredients in a recipe. Just as the recipe dictates the exact proportion of flour to sugar needed to get the perfect cookie, a balanced chemical equation tells you the precise ratio of moles of one substance to another needed for a reaction, ensuring everything combines correctly without leftovers.
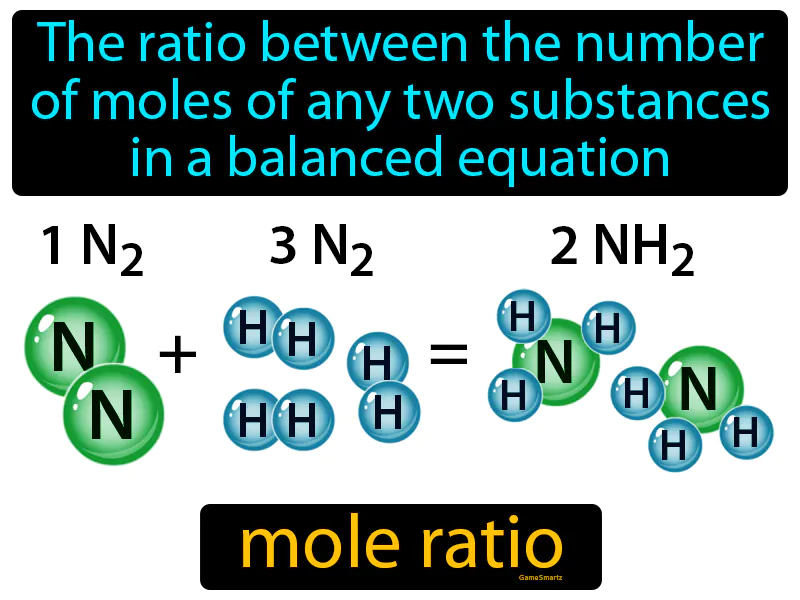
Practice Version
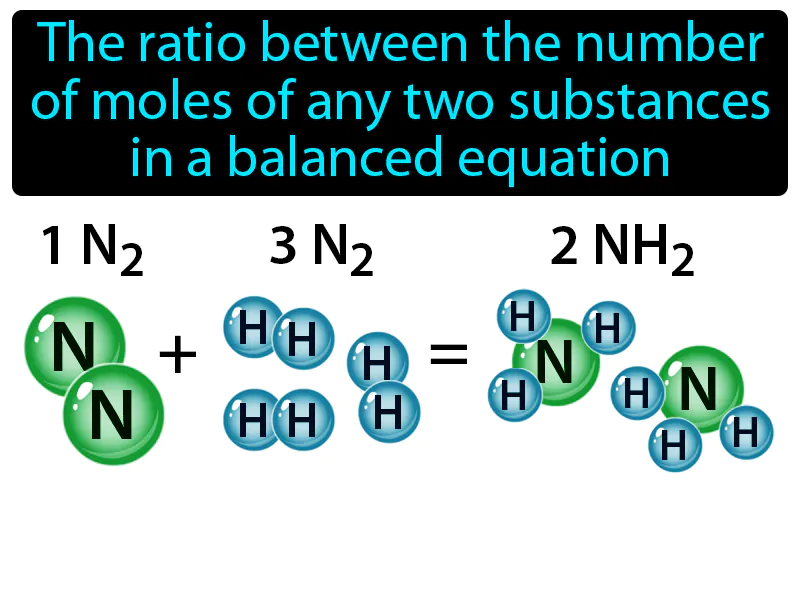
Mole Ratio: The ratio between the number of moles of any two substances in a balanced equation is called the mole ratio. In simple terms, a mole ratio tells you how many units of one substance react or are produced with units of another in a chemical reaction.