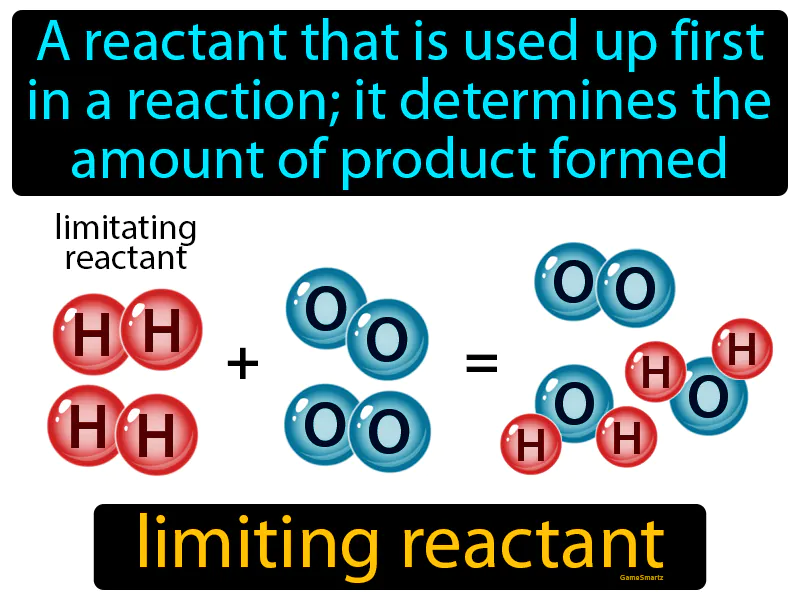
Limiting Reactant
Limiting Reactant: Easy to understand
Imagine you're planning to make sandwiches for a picnic, but you only have a limited number of slices of bread. In this scenario, much like in a chemical reaction, the number of sandwiches you can make is determined by the ingredient you run out of first, which is the bread. Here, the slices of bread are analogous to the limiting reactant in a chemical reaction; they dictate the maximum number of sandwiches (or product) you can create because once the bread is gone, it doesn't matter how much cheese or ham you have left—no more sandwiches can be made.
Practice Version
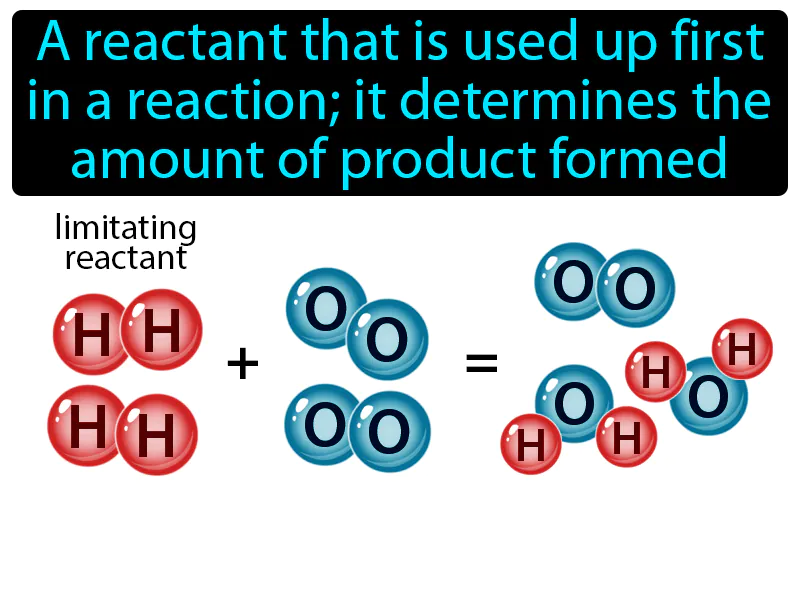
Limiting Reactant: A reactant that is used up first in a reaction it determines the amount of product formed. Limiting reactant. The limiting reactant is the substance in a chemical reaction that runs out first, stopping the reaction and determining how much product is made.