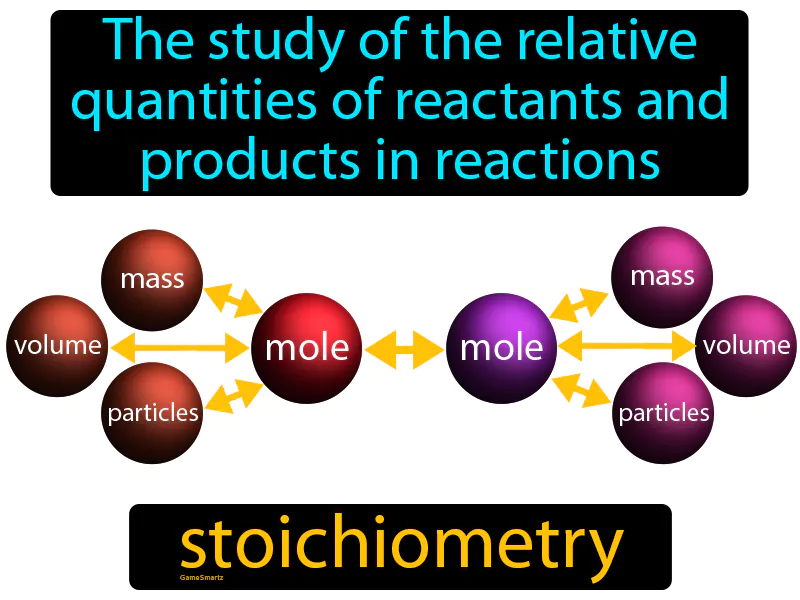
Stoichiometry
Stoichiometry: Easy to understand
Imagine you're planning a dinner party and need to determine how much of each ingredient is required to make enough lasagna for all your guests. This is similar to stoichiometry in chemistry, where you calculate the exact amounts of reactants needed to produce a desired quantity of products in a chemical reaction. Just like you need a precise recipe to ensure everyone at your party gets a piece of lasagna, stoichiometry ensures that chemical reactions have the right balance of ingredients (reactants) to produce the correct amount of outcome (products), making sure nothing is wasted or missing.
Practice Version
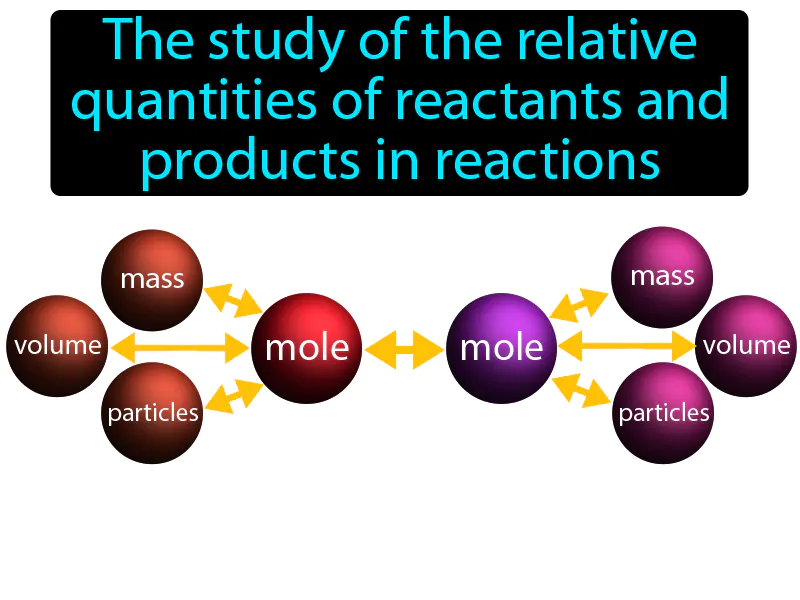
Stoichiometry: The study of the relative quantities of reactants and products in reactions. Stoichiometry. It is the calculation of the amounts of substances involved in chemical reactions.