Reduction Potential
Reduction Potential: Easy to understand
Imagine you're at a crowded party, and everyone is trying to decide which person to approach and talk to. Just like some people at the party naturally attract more attention and are more likely to be approached by others, some substances have a stronger tendency to gain electrons. In this analogy, the guests at the party represent electrons, and the people who naturally become the center of attention represent substances with a high reduction potential. Just as some individuals are more magnetic and draw people in, substances with higher reduction potentials are better at attracting and gaining electrons.
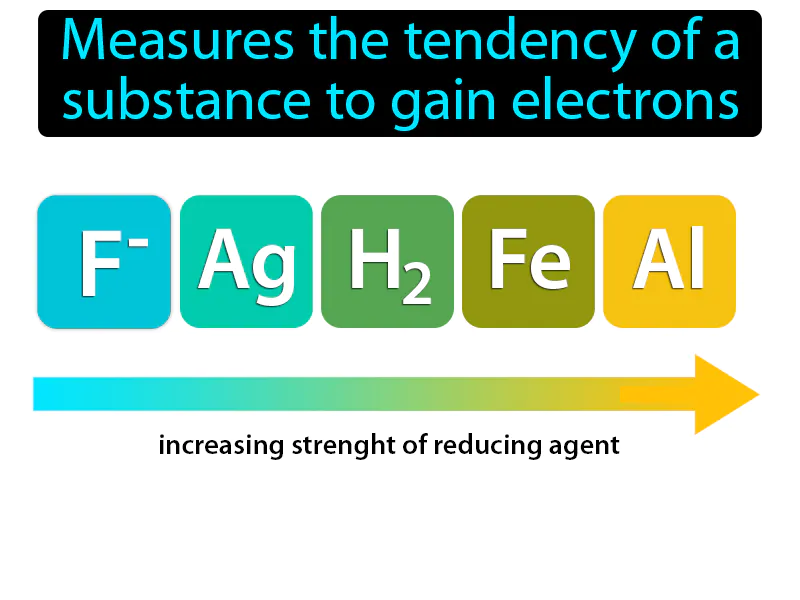
Practice Version
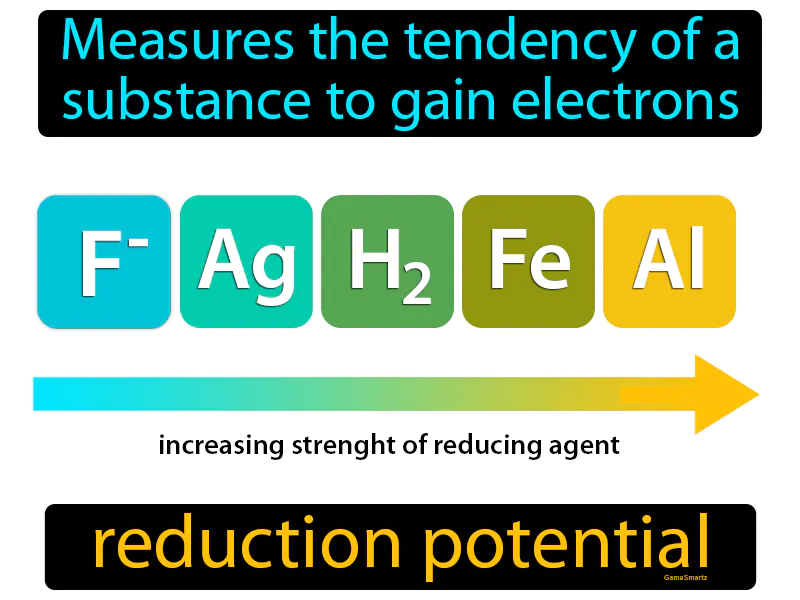
Reduction Potential: Measures the tendency of a substance to gain electrons. Reduction potential. Reduction potential is a measure of how likely a substance is to receive electrons in a chemical reaction.