Half-cell
Half-cell: Easy to understand
Imagine trying to have a conversation on the phone while the person on the other end is in a noisy environment. This situation is similar to a battery, where each electrode (or half-cell) must work in harmony with its pair to facilitate the flow of electrons, much like both ends of a phone call need to be clear for effective communication. Just as the phone call relies on both people being able to hear and speak clearly, a battery relies on both half-cells to balance and maintain a flow of electrons, ensuring that the energy can move efficiently from one electrode to another, completing the circuit.

Practice Version
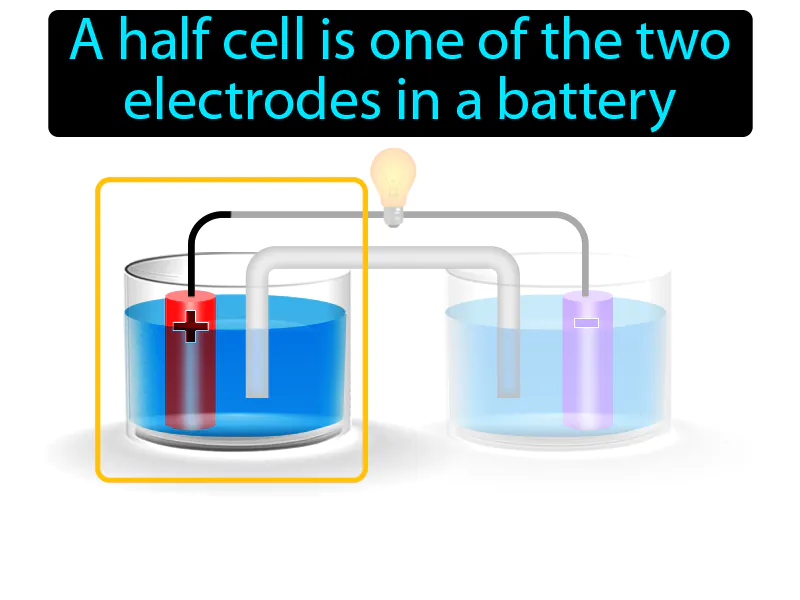
Half-cell: A half cell is one of the two electrodes in a battery. A half-cell is a part of a battery where either oxidation or reduction takes place.