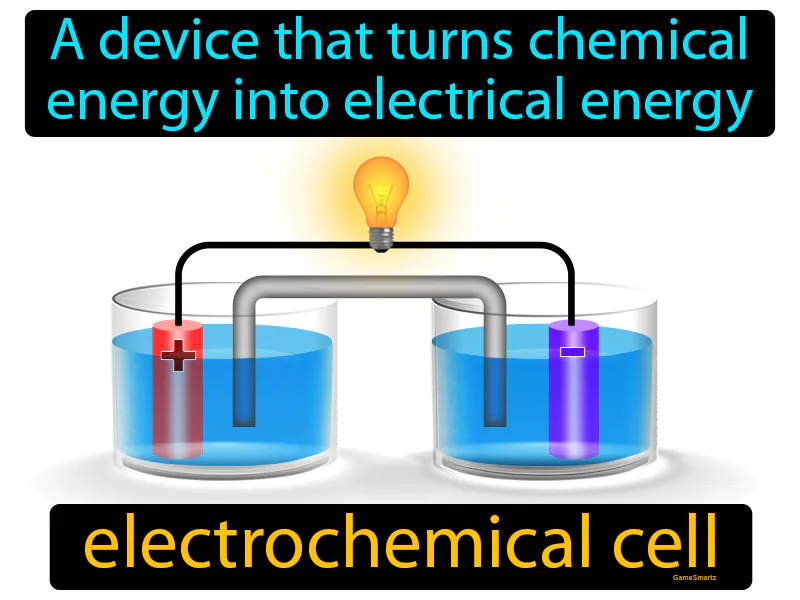
Electrochemical Cell
Electrochemical Cell: Easy to understand
Imagine trying to charge your phone with mismatched cables that don't fit properly. This scenario is similar to how an electrochemical cell operates, as both involve the challenge of transferring energy from one form to another efficiently. In the case of an electrochemical cell, the 'mismatched cables' are like the electrodes, where one fits perfectly to release electrons (like the anode) and the other to receive them (like the cathode), facilitating the flow of electrical energy from chemical reactions, just as a proper cable would allow your phone to charge effectively.
Practice Version
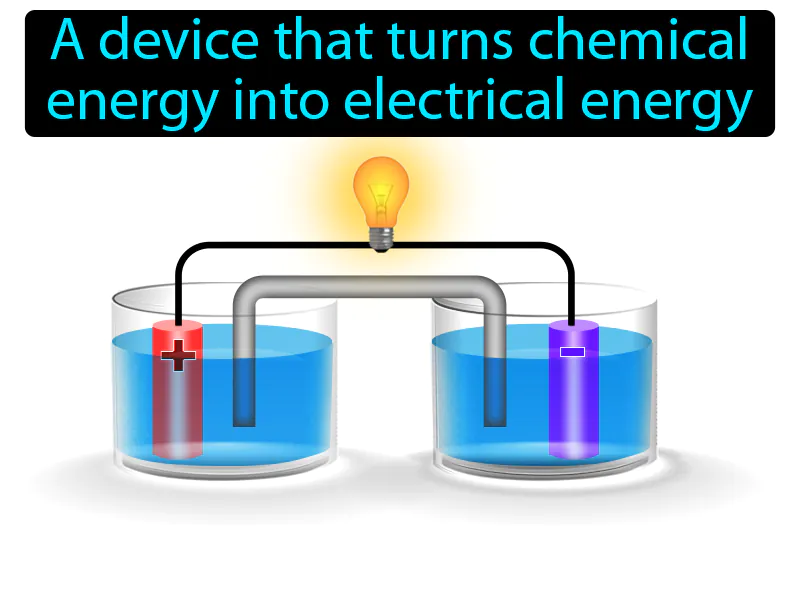
Electrochemical Cell: A device that turns chemical energy into electrical energy. Electrochemical cell. An electrochemical cell is a device that generates electricity through chemical reactions.