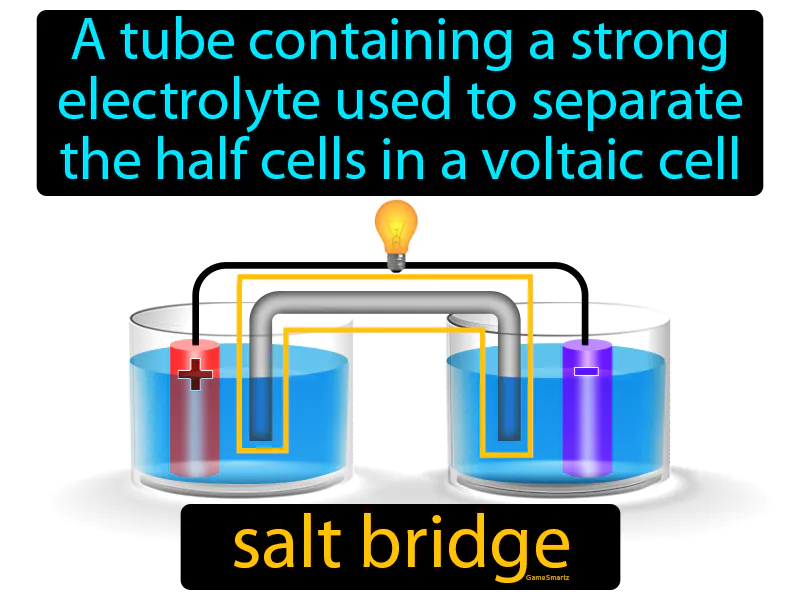
Salt Bridge
Salt Bridge: Easy to understand
Imagine you're at a party, and there's a dance floor with two groups of people, but nobody wants to cross the awkward empty space in between. A salt bridge in a voltaic cell acts like a friendly host at the party who encourages people to mingle and move between the two groups, making the atmosphere more lively. Just like the host ensures everyone interacts and the energy flows at the party, the salt bridge allows ions to flow between the half cells, maintaining electrical neutrality and enabling the flow of electrons from one side to the other.
Practice Version
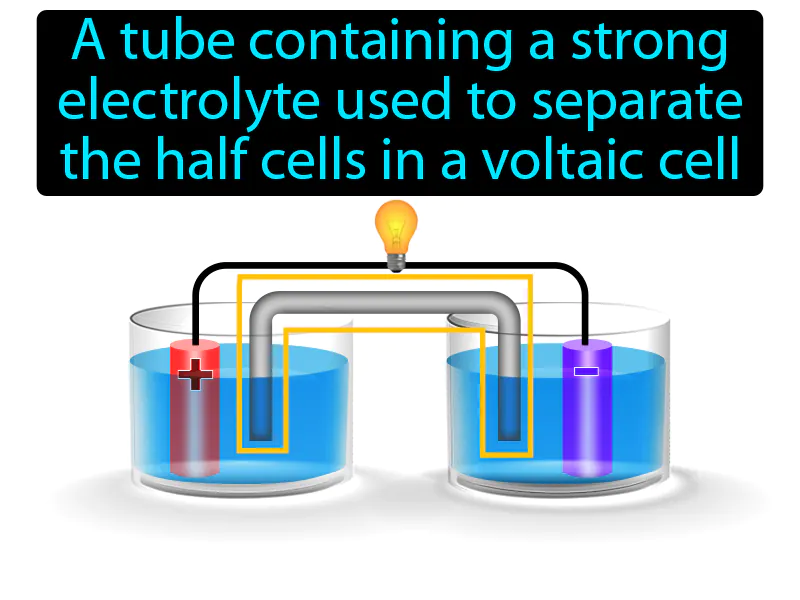
Salt Bridge: A tube containing a strong electrolyte used to separate the half cells in a voltaic cell. Salt bridge. A salt bridge is a device that allows ions to flow between two half-cells, maintaining electrical neutrality in a voltaic cell.