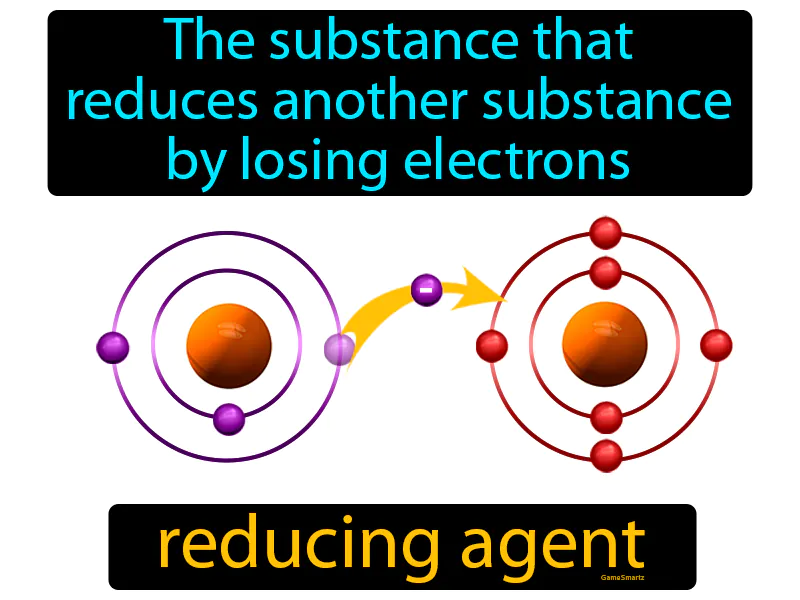
Reducing Agent
Reducing Agent: Easy to understand
Imagine you're at a crowded party, and everyone is trying to find a spot to sit, but there aren’t enough chairs. It's similar to how a reducing agent works in a chemical reaction, where it sacrifices its own 'comfort' by giving away its electrons to help another substance 'sit' in a more stable electron configuration. Just as you might offer your chair to someone else so they can sit down, a reducing agent gives up its electrons, allowing the other substance to become more stable by 'taking a seat' in a lower energy state.
Practice Version
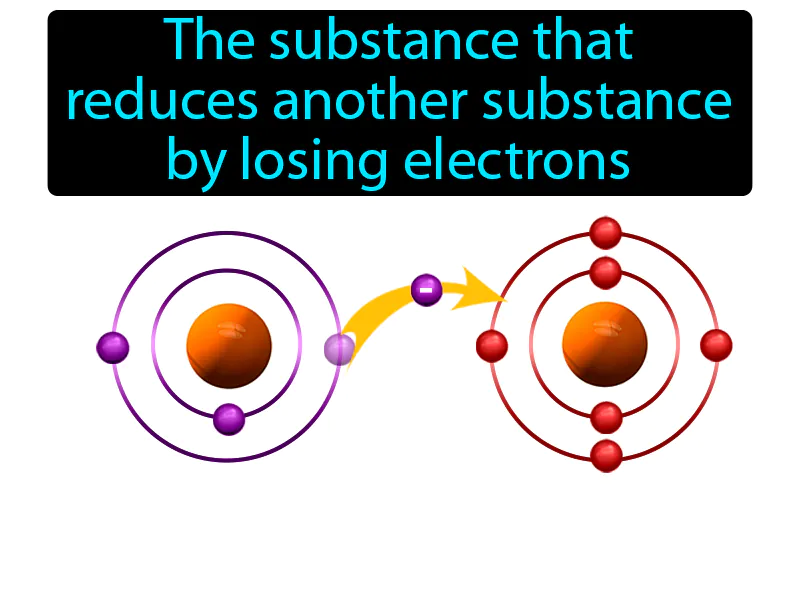
Reducing Agent: The substance that reduces another substance by losing electrons reducing agent. A reducing agent is a chemical that donates electrons to another substance in a reaction.