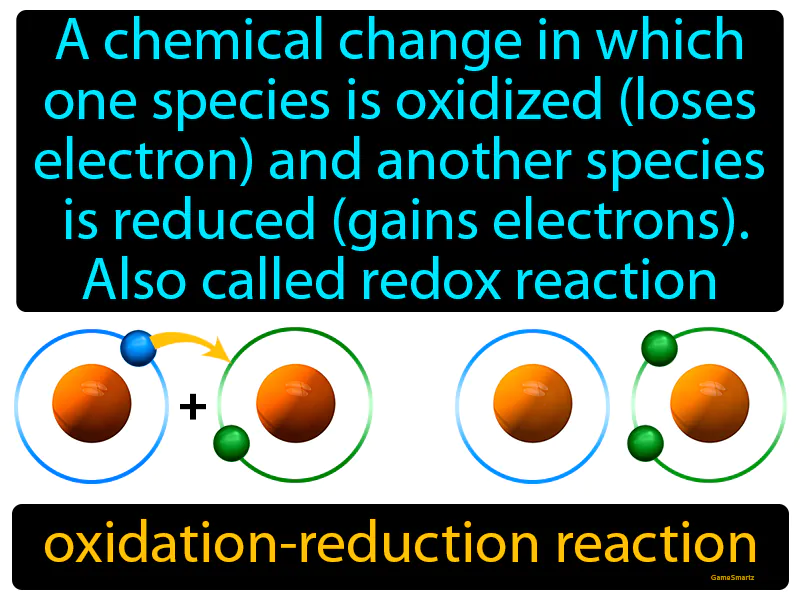
Oxidation-reduction Reaction
Oxidation-reduction Reaction: Easy to understand
Imagine you're at a party where everyone is trying to balance out their conversations; some people are talking too much, while others are too quiet. This scenario is similar to an oxidation-reduction (redox) reaction, where one species "talks too much" by losing electrons (is oxidized), while another becomes "more engaging" by gaining electrons (is reduced). Just as the social balance is restored when a chatterbox listens (loses "talk") and a quieter person speaks up (gains "talk"), in a redox reaction, the balance of electrons is adjusted as one species donates electrons and another accepts them.
Practice Version
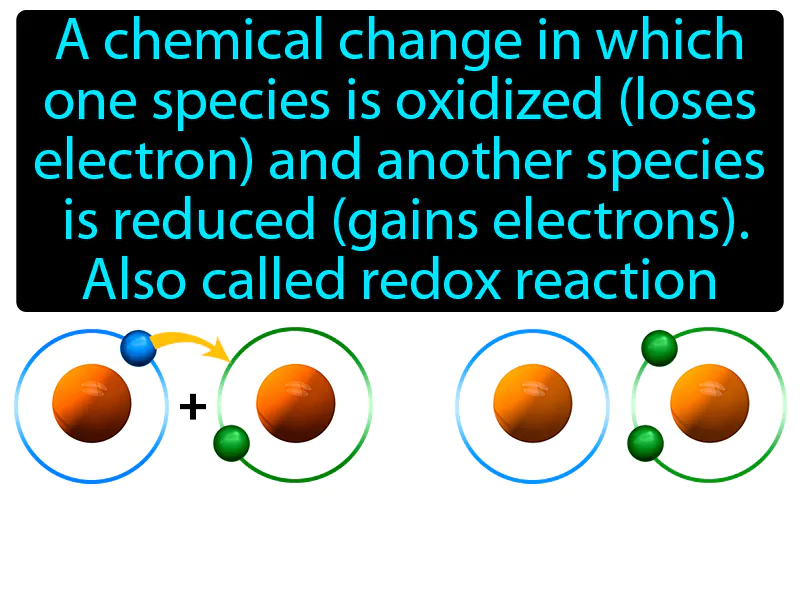
Oxidation-reduction Reaction: A chemical change in which one species is oxidized loses electrons and another species is reduced gains electrons, also called redox reaction. Oxidation-reduction reaction. In simple terms, a redox reaction is when one substance donates electrons to another, changing their electron counts.