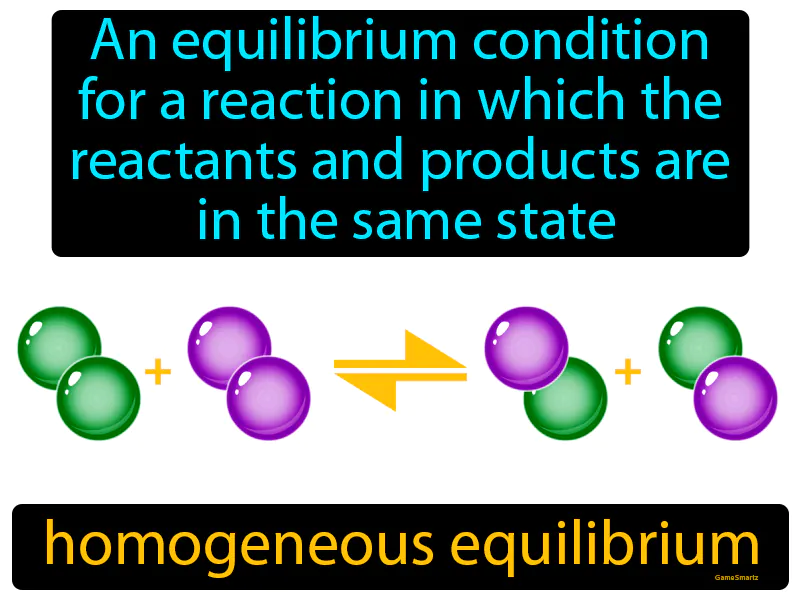
Homogeneous Equilibrium
Homogeneous Equilibrium: Easy to understand
Imagine you're trying to maintain a harmonious room temperature when multiple people in the room prefer different levels of warmth. Just like balancing everyone's comfort requires the heater and air conditioner to work in concert without changing the overall temperature, a homogeneous equilibrium in chemistry is when reactants and products coexist in the same state, maintaining a steady reaction condition. Here, the room's temperature is analogous to the state of the chemical system, where the heater and air conditioner represent the reactants and products working together to keep everything balanced and consistent.
Practice Version
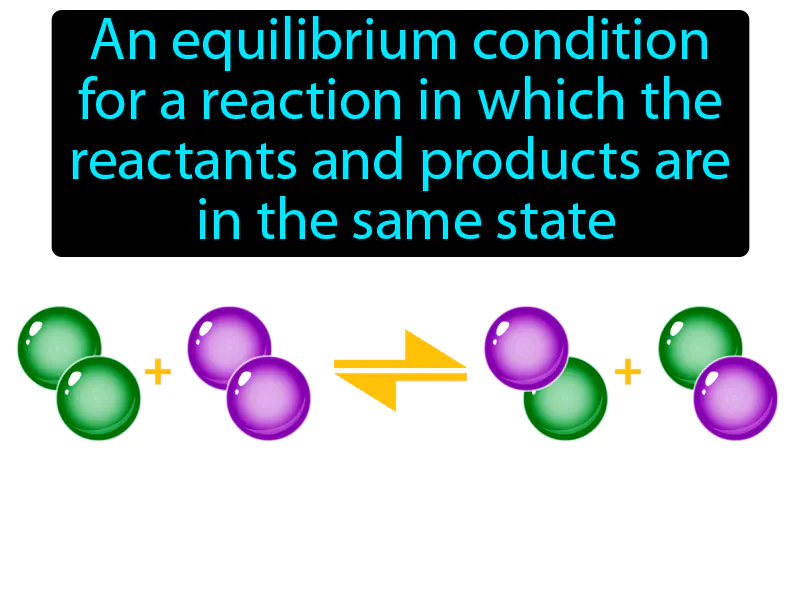
Homogeneous Equilibrium: An equilibrium condition for a reaction in which the reactants and products are in the same state, solid, liquid, or gas. Homogeneous equilibrium. It occurs when substances in a chemical reaction are all in the same physical state.