Common Ion Effect
Common Ion Effect: Easy to understand
Imagine you're trying to find a parking spot in an already crowded lot. Just as the lot has limited space, a solution can only hold a limited amount of a dissolved substance. When more cars (or ions) of the same make are added, it becomes harder to find a spot, just as adding more of the same ion reduces the solubility of a compound. In both cases, the presence of similar elements—whether cars or ions—limits the availability of space, be it parking or solubility.
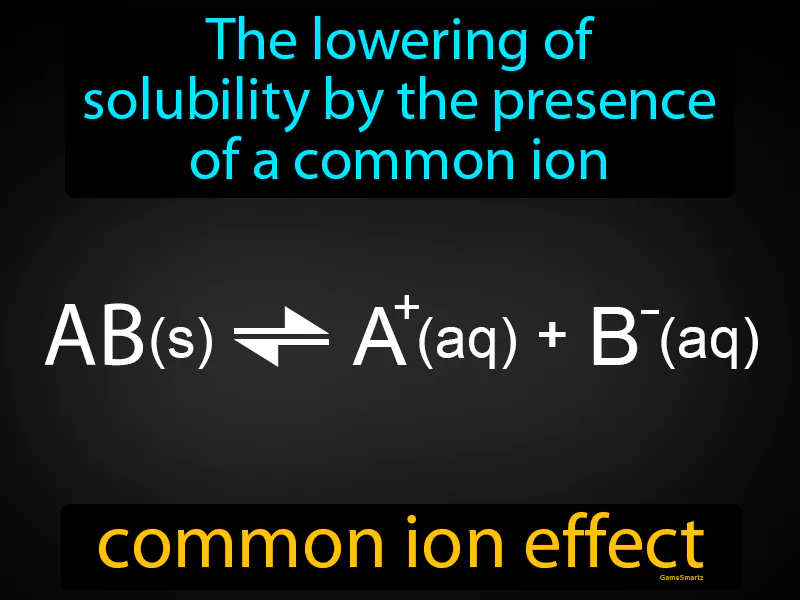
Practice Version
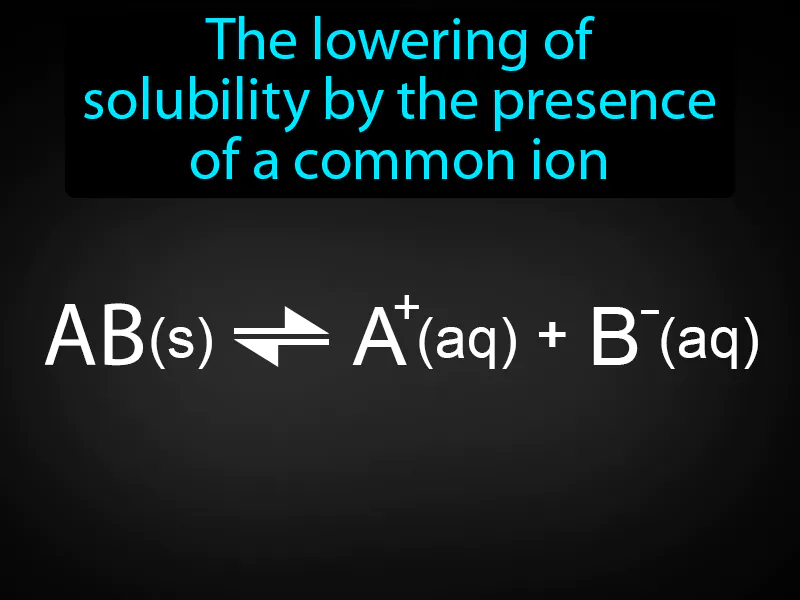
Common Ion Effect: The lowering of solubility by the presence of a common ion. Common ion effect. The common ion effect occurs when a compound's solubility decreases due to the presence of another compound that shares a common ion in solution.