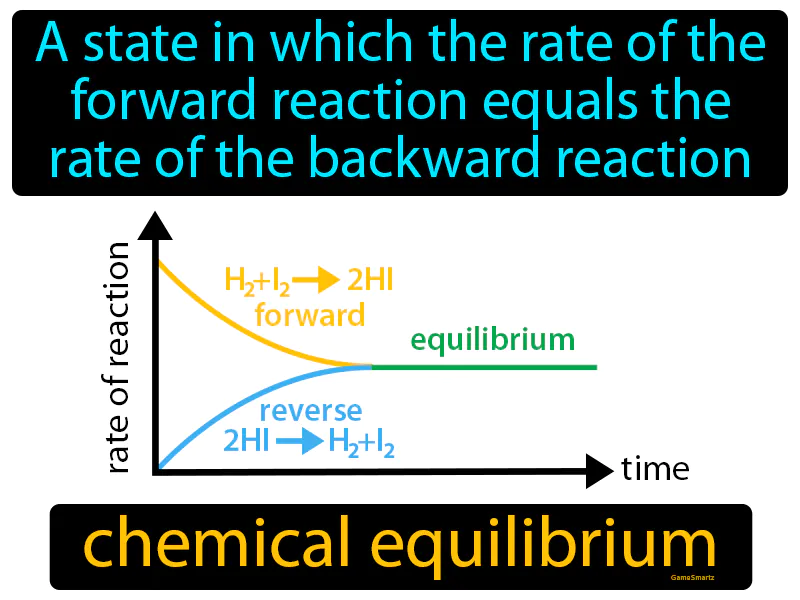
Chemical Equilibrium
Chemical Equilibrium: Easy to understand
Imagine you're trying to maintain a stable room temperature by adjusting a heater and an air conditioner. This situation is similar to chemical equilibrium, where the forward reaction (adding heat) and the backward reaction (removing heat) balance each other out. Just as the heater and air conditioner work at the same rate to keep the room temperature constant, in chemical equilibrium, the rate of the forward reaction equals the rate of the backward reaction, keeping the concentrations of reactants and products stable.
Practice Version

Chemical Equilibrium: A state in which the rate of the forward reaction equals the rate of the backward reaction. Chemical equilibrium. Chemical equilibrium is when a chemical reaction has balanced out so that its products and reactants remain constant over time.