Lanthanide Series
Lanthanide Series: Easy to understand
Imagine you're trying to remember the order of items on a long grocery list, where each item seems very similar to the next. This is much like the lanthanide series, a group of 14 f-block elements that closely resemble each other in properties and follow lanthanum on the periodic table. Just as each grocery item has subtle differences that help distinguish it from the rest, each lanthanide element has slight variations in electron configuration and atomic number, helping scientists differentiate them despite their overall similarity.
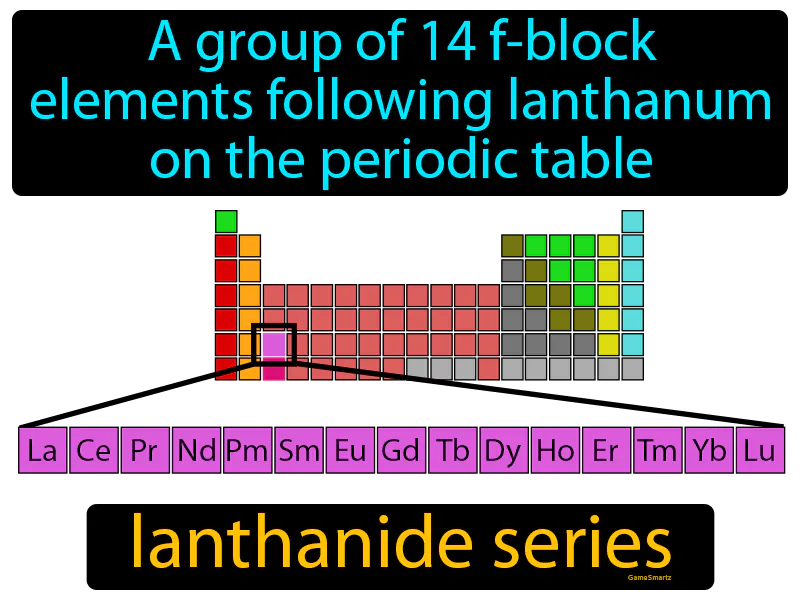
Practice Version
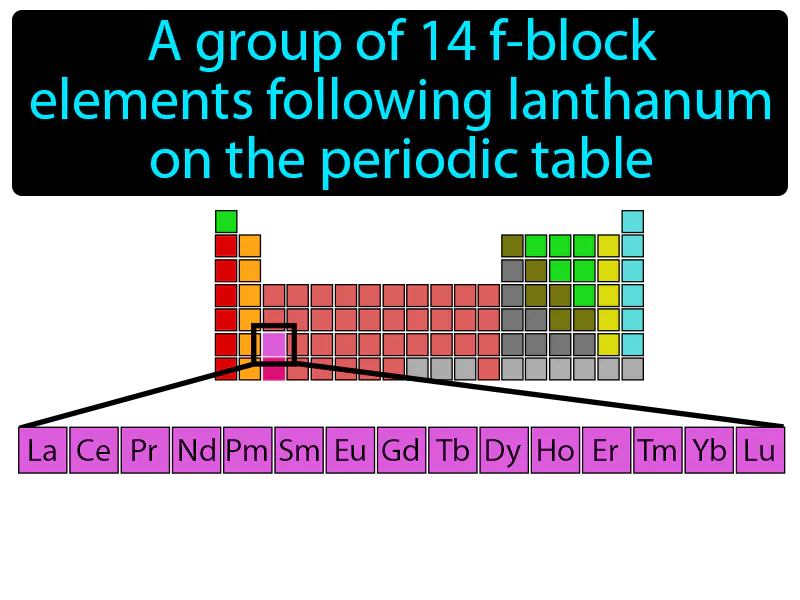
Lanthanide Series: A group of 14 f-block elements following lanthanum on the periodic table. Lanthanide series. The lanthanide series consists of elements with atomic numbers 58 to 71 that are shiny and often used in electronics.