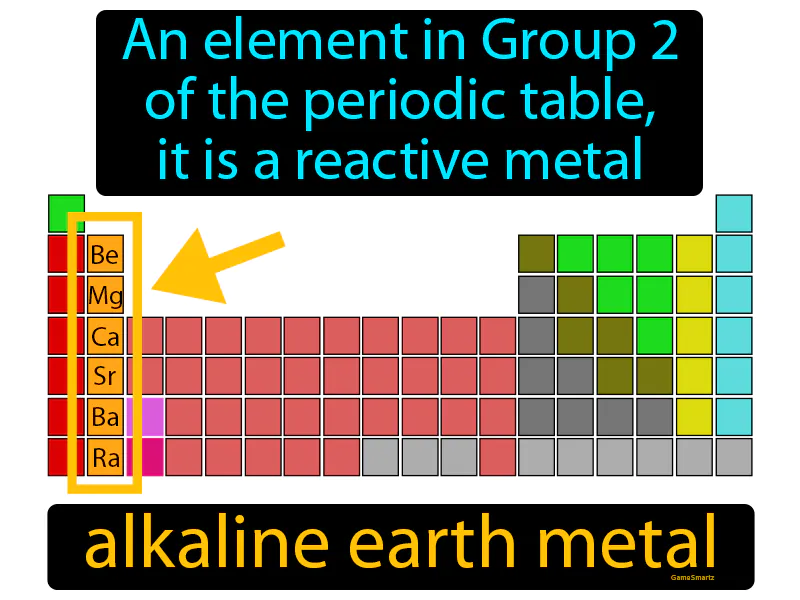
Alkaline Earth Metal
Alkaline Earth Metal: Easy to understand
Imagine trying to carry groceries in a paper bag that gets soggy in the rain. Just like the paper bag quickly reacts to moisture and becomes compromised, alkaline earth metals, such as magnesium and calcium, are highly reactive, especially with water. The paper bag's tendency to weaken and break when wet mirrors how alkaline earth metals readily react to form compounds, highlighting their reactive nature and the importance of handling them carefully, much like protecting your groceries from the rain.
Practice Version
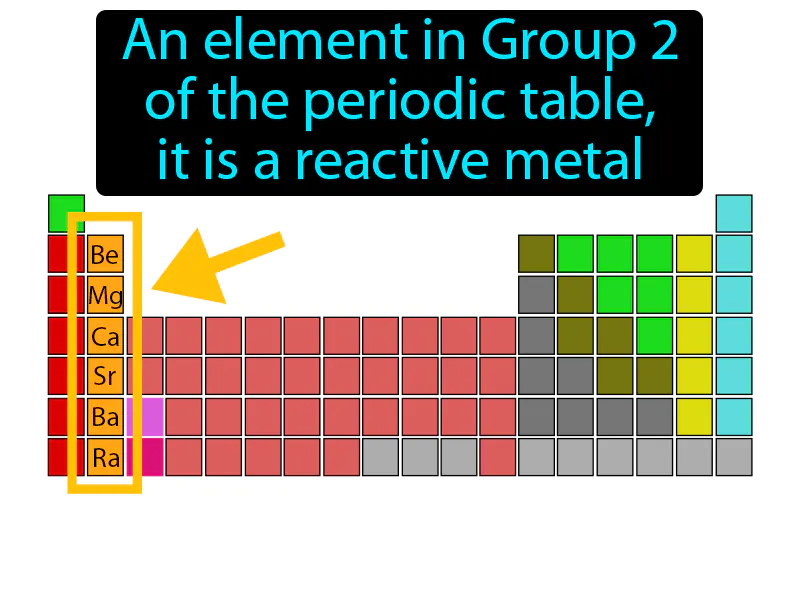
Alkaline Earth Metal: An element in Group 2 of the periodic table. It is a reactive metal. Alkaline earth metals are a group of shiny, silvery metals that are highly reactive and are commonly found in various minerals.