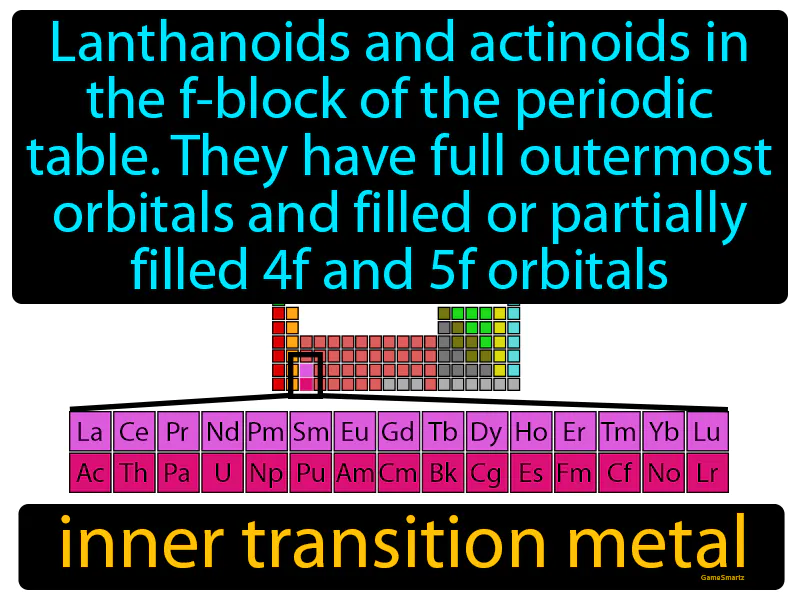
Inner Transition Metal
Inner Transition Metal: Easy to understand
Imagine trying to organize a cluttered attic where you have boxes stacked in layers, some fully packed and others only partially filled. This is similar to how lanthanoids and actinoids in the f-block of the periodic table have their outermost orbitals full while the 4f and 5f orbitals are either filled or partially filled. Just as you need to decide which boxes to sort through first based on their contents, chemists focus on the 4f and 5f orbitals because their varying levels of occupancy influence the chemical properties of these elements, much like how the attention you give to different boxes determines the overall organization of your attic.
Practice Version
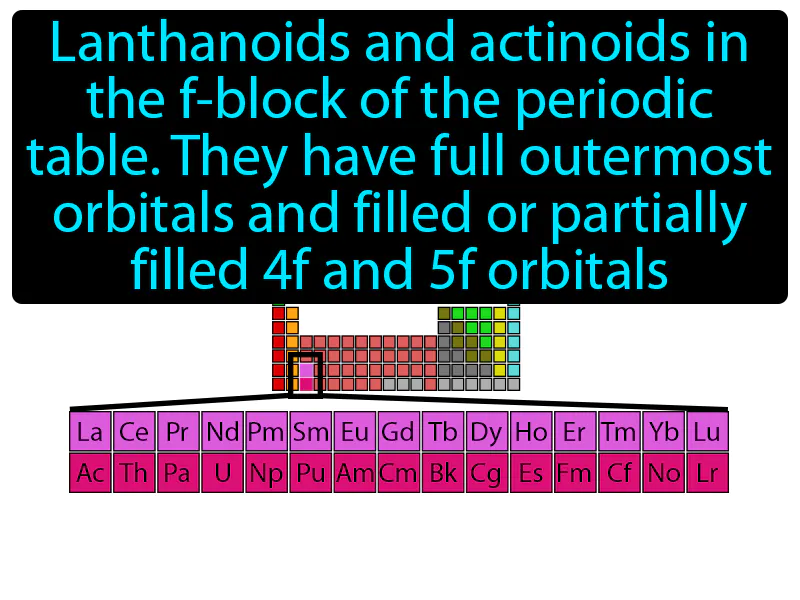
Inner Transition Metal: Lanthanoids and actinoids in the f-block of the periodic table, they have full outermost orbitals and filled or partially filled 4f and 5f orbitals. Inner transition metals are elements in the f-block of the periodic table that have electrons filling their inner f orbitals.