Law Of Definite Proportion
Law Of Definite Proportion: Easy to understand
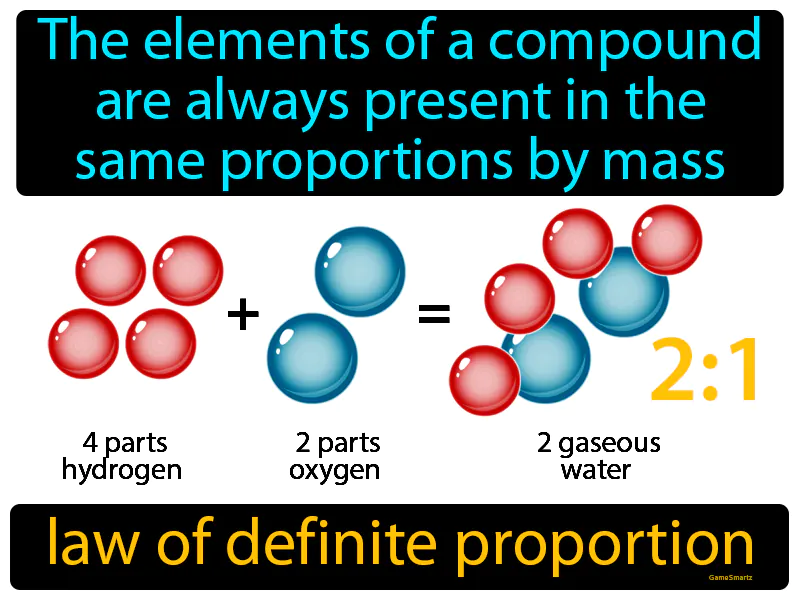
Imagine baking a cake and realizing that every time you do, you must use the exact same ratio of ingredients to get the perfect result. This is similar to how the law of definite proportions states that elements in a chemical compound are always combined in the same fixed ratios by mass, ensuring consistency in the compound's properties. Just as a cake requires precise amounts of flour, sugar, and eggs every time to taste the same, a compound like water always consists of hydrogen and oxygen in a fixed mass ratio, ensuring it remains consistent regardless of where or how it's produced.
Practice Version
Law Of Definite Proportion: The elements of a compound are always present in the same proportions by mass. Law of definite proportion. This law means that no matter how much of a compound you have, the ratio of its elements by weight is constant and unchanging.