Law Of Conservation Of Mass
What is the Law of Conservation of Mass? Explained in an easy to understand way:
What's Covered in the Video:
Imagine you're trying to rearrange the furniture in your living room to make it look more spacious. Just like in rearranging the furniture, where you can't change the total amount of furniture you have, in a chemical reaction, you can't change the total mass of the substances involved. In both scenarios, whether with furniture or with chemical reactions, you're simply redistributing what's already there without adding or removing anything—essentially, the total 'stuff' remains constant, illustrating the law of conservation of mass.
Practice Version
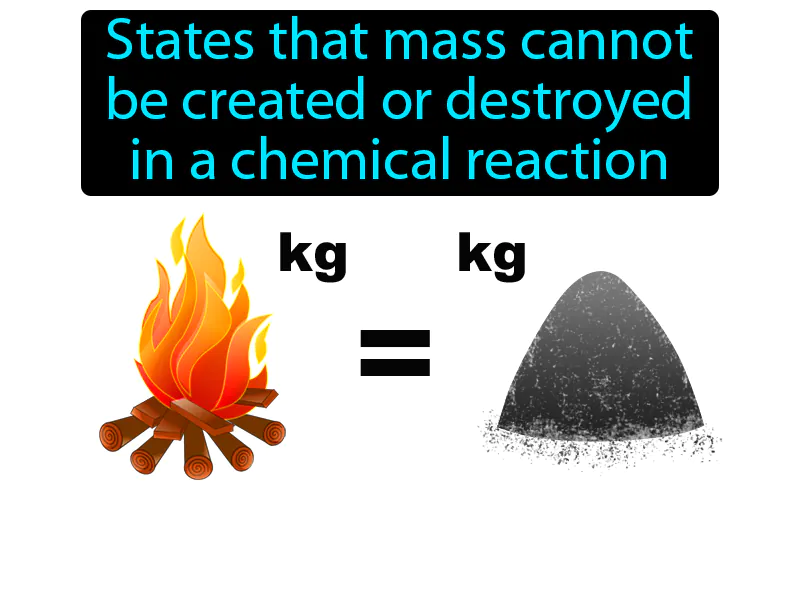
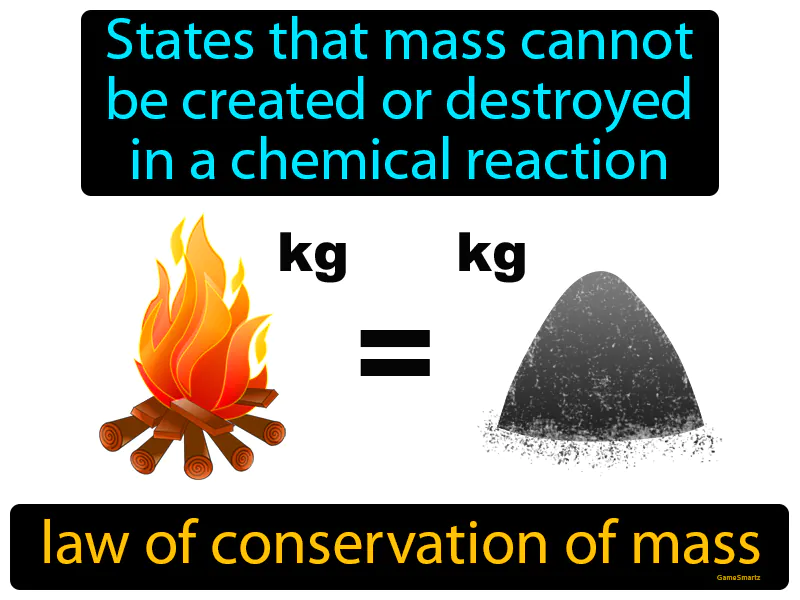
Law Of Conservation Of Mass: States that mass cannot be created or destroyed in a chemical reaction. Law of conservation of mass. It means that in a chemical reaction, the total mass of the substances involved stays the same.