Mass Defect
Mass Defect: Easy to understand
Imagine trying to pack all your groceries into a single bag, only to find that the bag feels lighter than if you were to add up the weight of each item separately. This is similar to the mass defect in physics, where the combined mass of an atom is less than the sum of its individual protons, neutrons, and electrons. Just as the act of arranging groceries efficiently in the bag makes it feel lighter due to the way they fit together, the binding energy that holds an atom's nucleus together reduces its total mass, making the whole atom lighter than the sum of its parts.
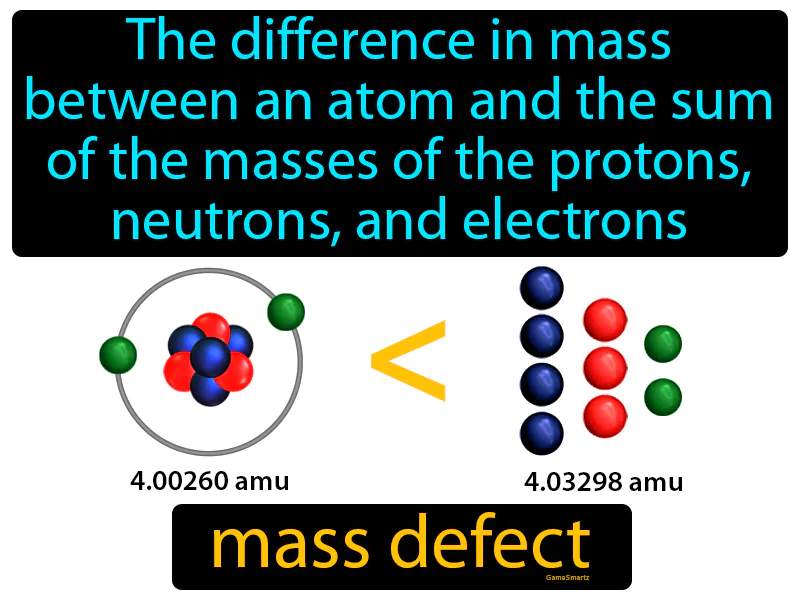
Practice Version
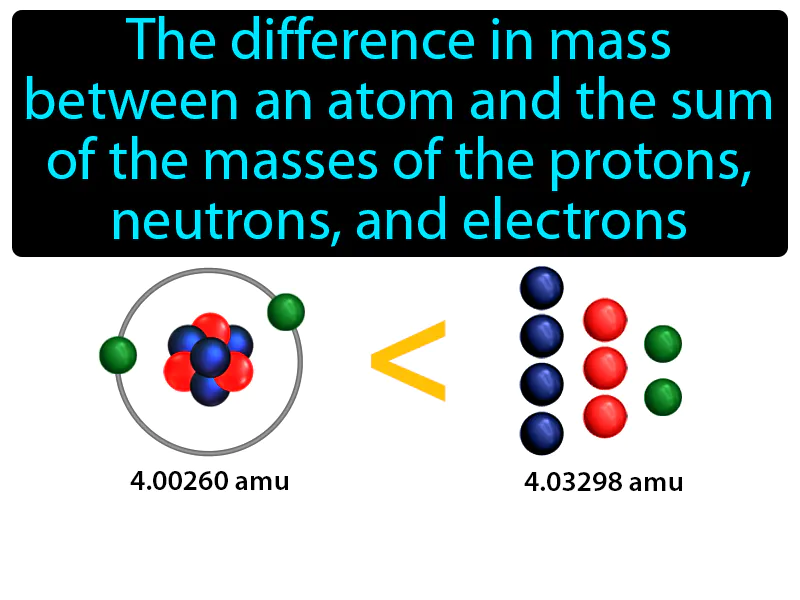
Mass Defect: The difference in mass between an atom and the sum of the masses of the protons, neutrons, and electrons mass defect. Mass defect is the small amount of mass lost and converted into energy when atomic nuclei are formed.