VSEPR Model
VSEPR Model: Easy to understand
Imagine trying to arrange a group photo where everyone wants to stand apart from each other as much as possible to avoid awkwardness. This is similar to how electrons behave in the VSEPR model, where electrons in a molecule repel each other, arranging themselves to be as far apart as possible. Just as people in the photo spread out to minimize uncomfortable closeness, electron pairs in a molecule spread out to minimize repulsion, which ultimately determines the shape of the molecule.
Practice Version
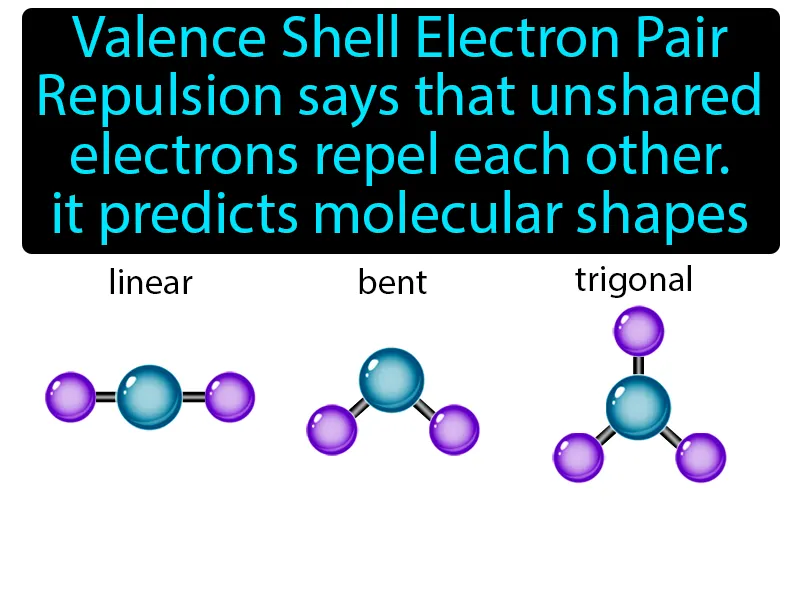
VSEPR Model: Valence Shell Electron Pair Repulsion says that unshared electrons repel each other, it predicts molecular shapes. VSEPR model. The VSEPR model helps determine the 3D shape of molecules by considering how electron pairs push each other apart.