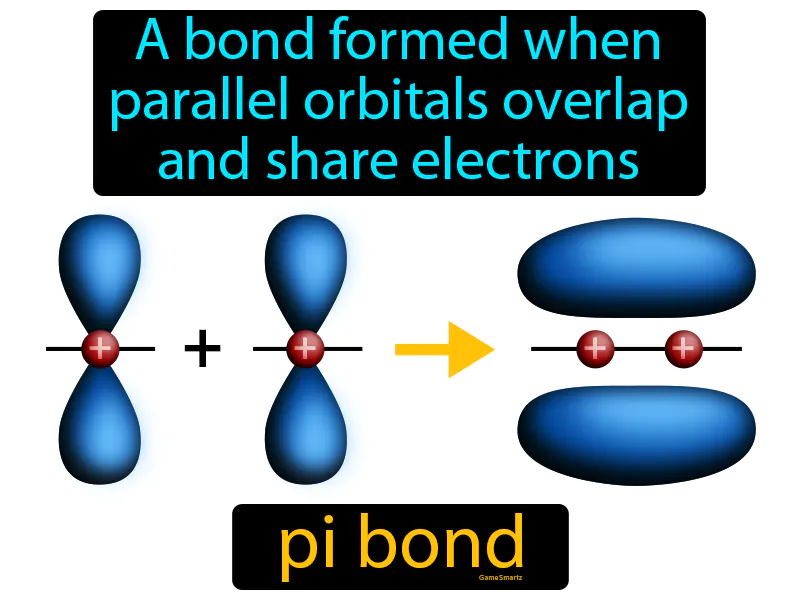
Pi Bond
Pi Bond: Easy to understand
Imagine you're trying to hang a picture frame on a wall, but the wall is made of soft material, so nails won't hold the frame securely. This is similar to how pi bonds form when parallel orbitals overlap and share electrons, creating a bond that isn't as strong as a sigma bond but still holds the atoms together. Just as you might use a special adhesive to spread across the wall's surface to hold the frame, pi bonds use the sideways overlap of orbitals to establish a stable connection between atoms, even when direct, end-to-end bonding isn't possible.
Practice Version
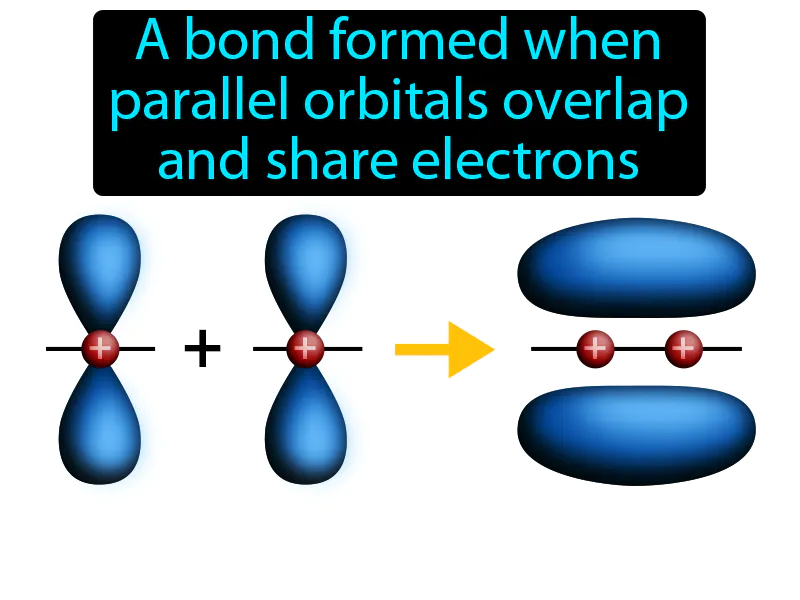
Pi Bond: A bond formed when parallel orbitals overlap and share electrons. Pi bond. A pi bond is a type of chemical bond where electrons are shared in overlapping orbitals, typically above and below the bonding atoms.