Supersaturated Solution
What is a Supersaturated Solution? Explained in an easy to understand way:
What's Covered in the Video:
Imagine trying to pack a suitcase for a long trip, and you've already filled it to capacity with clothes, but you keep trying to squeeze in more items by pressing down on the lid. This scenario is similar to a supersaturated solution where the liquid holds more dissolved solute than it should at a given temperature, much like the overpacked suitcase. Just as the suitcase has been pushed beyond its usual limits to hold extra clothes, a liquid in a supersaturated state contains more solute than it would under normal circumstances, waiting for a slight disturbance – like opening the suitcase lid – to overflow or crystallize.
Practice Version
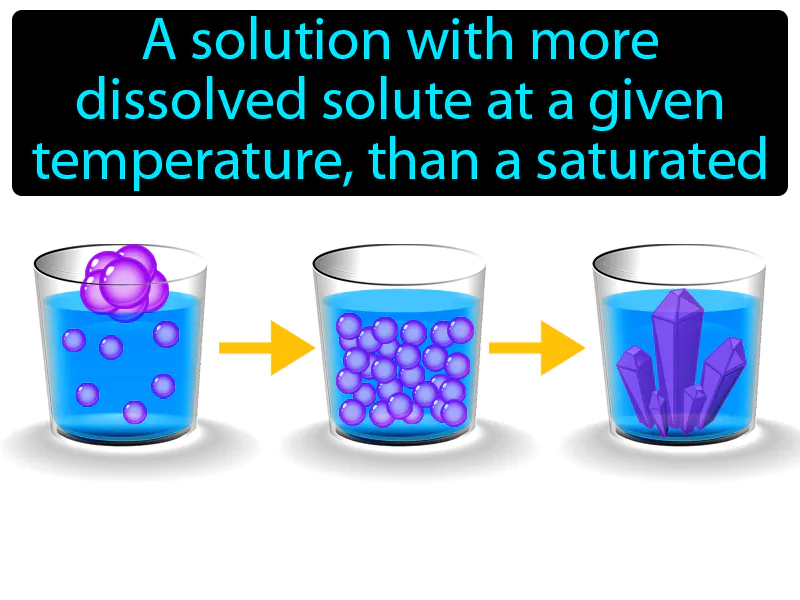
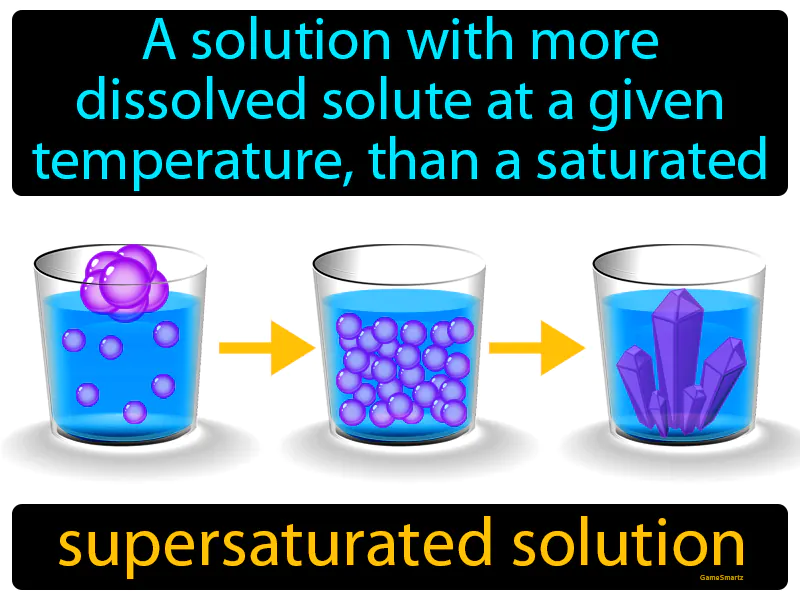
Supersaturated Solution: A solution with more dissolved solute at a given temperature, than a saturated solution. Supersaturated solution. A supersaturated solution contains more dissolved material than it normally would hold at a given temperature, and is unstable.