Saturated Solution
What is a Saturated Solution? Explained in an easy to understand way:

What's Covered in the Video:
Imagine trying to pack a suitcase for a long trip, and you find that you've reached the point where no matter how much you squeeze and rearrange, you just can't fit in another item. This situation is similar to a saturated solution, where the solution has dissolved as much solute as it can at a given temperature, and adding more solute will result in undissolved particles, just like trying to add more clothes to your already full suitcase. In this analogy, the suitcase represents the solvent, the clothes are the solute, and the moment when no more clothes can fit equates to the solution reaching its saturation point.

Practice Version
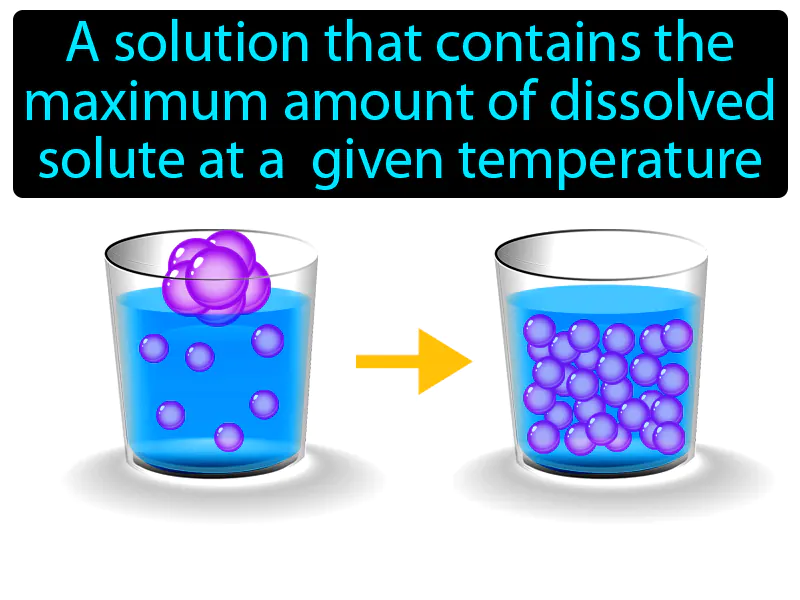
Saturated Solution: A solution that contains the maximum amount of dissolved solute at a given temperature. Saturated solution. A saturated solution is one where no more solute can dissolve in the liquid at that specific temperature.