Catalyst
What is a Catalyst? Explained in an easy to understand way:
What's Covered in the Video:
Imagine you're trying to push a heavy shopping cart over a speed bump in a parking lot. Just as a catalyst helps a chemical reaction by lowering the activation energy, a friend giving a gentle push can help you move the cart over the bump more easily. In this analogy, the speed bump represents the activation energy barrier, your friend's push represents the catalyst, and the smoother movement of the cart is akin to the faster chemical reaction.
Practice Version

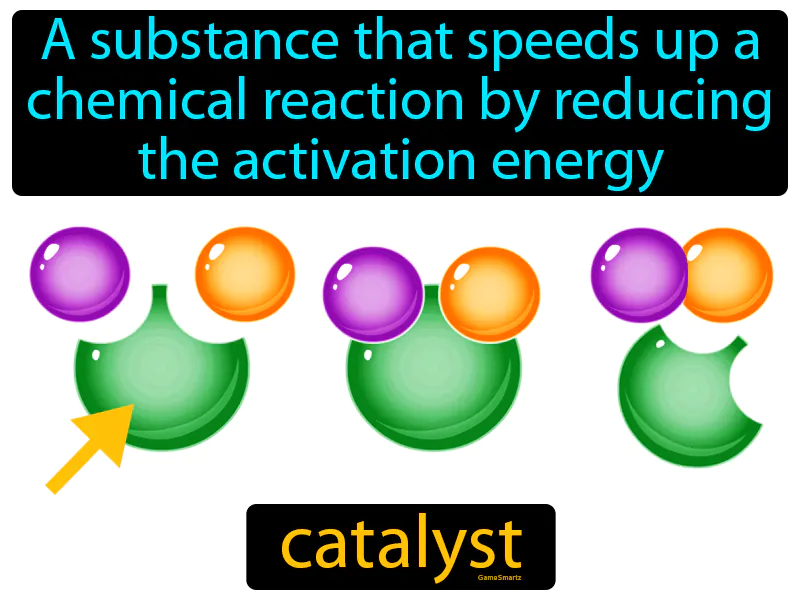
Catalyst: A substance that speeds up a chemical reaction by reducing the activation energy is called a catalyst. A catalyst makes a chemical reaction happen faster without being used up in the process.