Freezing Point Depression
Freezing Point Depression: Easy to understand
Imagine trying to walk through a crowded room versus an empty one. Just as moving freely is easier in an empty room, a pure solvent freezes at a higher temperature compared to when it's mixed with a solute, which disrupts its orderly freezing process. The crowded room represents the solute particles in a solution, which interfere with the solvent particles' ability to align and solidify, thereby lowering the freezing point compared to the pure, empty room where particles can freeze more easily.
Practice Version
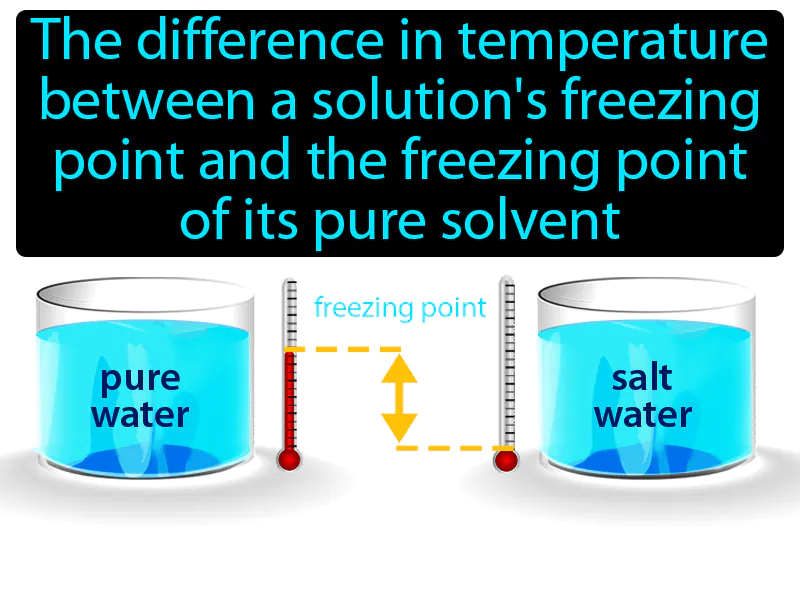
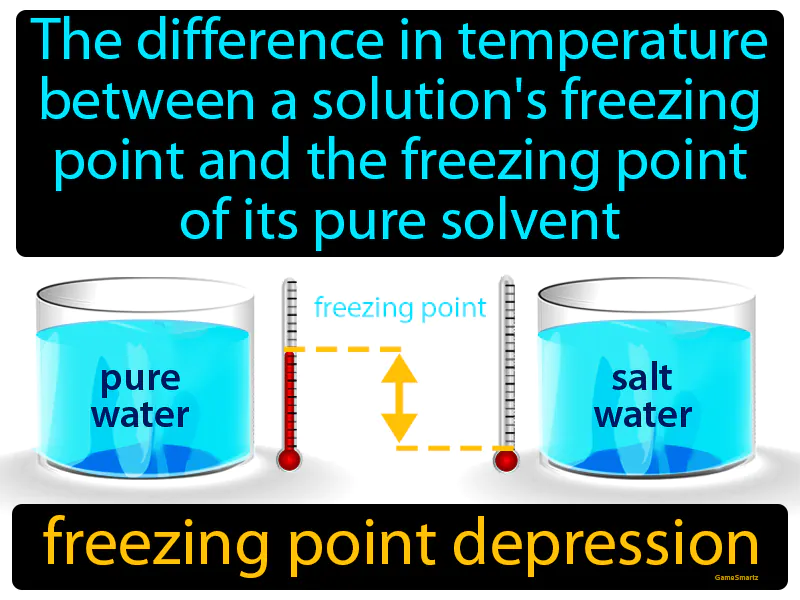
Freezing Point Depression: The difference in temperature between a solution's freezing point and the freezing point of its pure solvent. Freezing point depression. Freezing point depression is when adding a substance to a solvent lowers the temperature at which the liquid freezes.