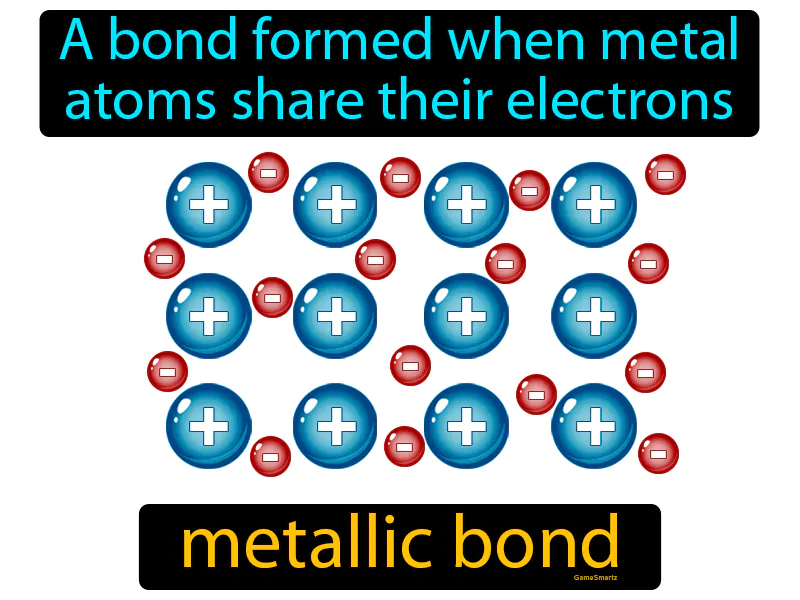
Metallic Bond
What is a Metallic Bond? Explained in an easy to understand way:

What's Covered in the Video:
Imagine you're at a crowded concert where everyone is trying to pass around a limited number of glow sticks to keep the party going. Just like the concertgoers sharing glow sticks to stay lively, metal atoms share electrons to maintain a stable structure. In this analogy, the concertgoers are the metal atoms, the glow sticks are the electrons, and the lively atmosphere represents the strong, cohesive force of a metallic bond that holds everything together.
Practice Version
Metallic Bond: A bond formed when metal atoms share electrons. Metallic bond. A metallic bond is when metal atoms share free electrons, allowing them to conduct electricity and heat.